Research
Current Research Projects
The main research theme in our group is discovering new electronic properties in organic and hybrid materials. En route to this goal, we are engaged in design and synthesis of novel pi-conjugated molecules and polymers, and study of their optical and electronic behavior, in solution, in thin films and crystals, and in semiconducting devices. Organic synthesis accounts for about 70% of our research activity, but the essence of these efforts is “making” novel properties, not merely new molecules. Thus, the new members of the group should also expect to develop expertise in one (or more) of the following: quantum-chemical calculations; supramolecular chemistry; photophysical and electrochemical measurements; semiconducting device fabrication; thin films and surface characterization techniques, scanning probe microscopy.
Some current research directions of the group, with examples of recent projects are given below:
Novel pi-Conjugated Systems
Extended pi-electron conjugation in polycyclic (hetero)aromatic molecules and conjugated polymers gives rise to a spectrum of special electronic and optical properties, including non-linear optical effects, electroluminescence, high electrical conductivity and magnetism. Through DFT-guided design and synthesis of novel pi-electron systems, we develop an understanding how these properties can be tuned and tailoring to specific applications, trying to push the limits of organic electronics through discovery of new electronic behavior in our materials.
Below are a few examples of conjugated molecular and polymeric systems designed and synthesized in our lab.
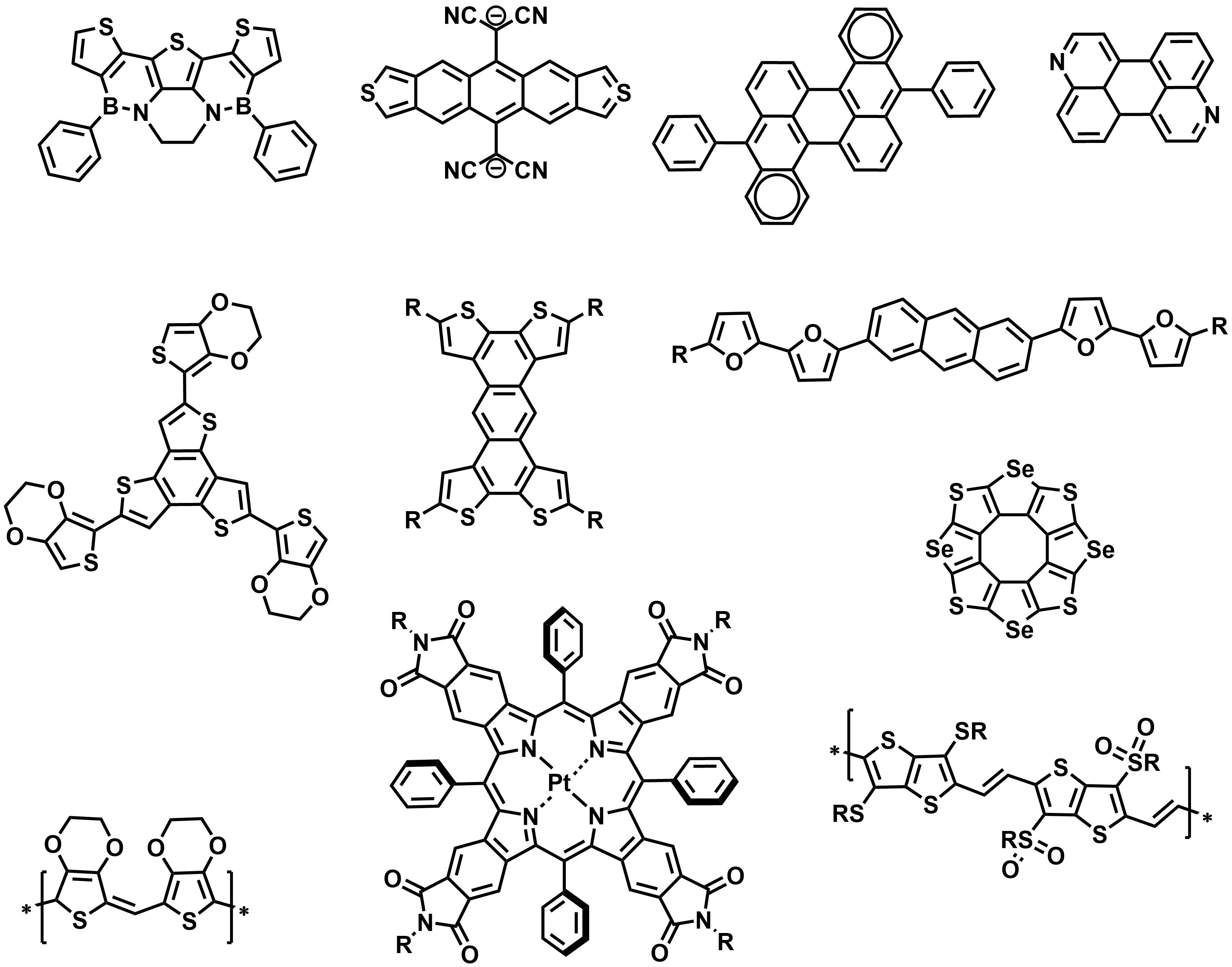
Some of the questions motivating the design and synthesis of these structures were:
• How low HOMO-LUMO gaps can be realized in a stable organic molecule and what would be the properties of such molecule?
• How does the topology of pi-conjugation (linear vs star-like or cross-like vs fully two-dimensional) affects the molecular properties?
• What controls the fluorescence quantum yield in organic molecules?
• How do heteroatoms affect the conductive and emissive properties of pi-conjugated materials?
• How does the length and parity of alkyl chains affects the packing of pi-conjugated molecules and their conducting properties?
Selected Publications
-
Aromatization of Benzannulated Perylene-3,9-diones: Unexpected Photophysical Properties and Reactivity,
M. R. Rao, S. Johnson, D. F. Perepichka, Org. Lett. 2016, 18, 3574–3577
A new approach to polycyclic azaarenes: visible-light photolysis of vinyl azides in the synthesis of diazabenzopyrene and diazaperylene,
J. A. Schneider, D. F. Perepichka, J. Mater. Chem. C 2016, 4, 7269-7276
Synthesis and Divergent Electronic Properties of Two Ring-Fused Derivatives of 9,10-Diphenylanthracene,
M. R. Rao, H. T. Black, D. F. Perepichka, Org. Lett. 2015, 17, 4224–4227
p-Extended Indenofluorenes,
M. R. Rao, A. Desmecht, D. F. Perepichka, Chem. Eur. J. 2015, 21, 6193-6201
Pentacenodithiadiazoledione, an n-type semiconductors for field effect transistors,
Z. Shi, H. T. Black, A. Dadvand, D. F. Perepichka, J. Org. Chem. 2014, 79, 5858–5860
Dithienonaphthothiadiazole Semiconductors: Synthesis, Properties, and Application to Ambipolar Field Effect Transistors,
Q. Shuai, H. T. Black, A. Dadvand, D. F. Perepichka, J. Mater. Chem. C. 2014, 2, 3972–3979
Tuning the Electronic Properties of Poly(thienothiophene vinylene)s via Alkylsulfanyl and Alkylsulfonyl Substituents,
J. A. Schneider, A. Dadvand, W. Wen, D. F. Perepichka, Macromolecules 2013, 46, 9231–9239
Convenient Synthesis of a Highly Soluble and Stable Phosphorescent Platinum Porphyrin Dye,
Y. Zems, A. Moiseev, D. F. Perepichka, Org. Lett. 2013, 15, 5330–5333
1,5-, 2,6- and 9,10-Distyrylanthracenes as luminescent organic semiconductors,
A. Dadvand, A. G. Moiseev, W.-H. Sun, F. Bélanger-Gariépy, F. Rosei, H. Meng, D. F. Perepichka, J. Mater. Chem. C 2013, 1, 2817-2825
Non-classical heteroacenes: synthesis and properties of anthra[2,3-c:6,7-c']dithiophene derivatives,
B. Djukic, D. F. Perepichka, Chem. Commun. 2011, 47, 12619-12621
New stable donor-acceptor dyads for molecular electronics,
M. Kondratenko, A. Moiseev, D. F. Perepichka, J. Mater. Chem. 2011, 21, 1470–1478
Donor-Acceptor Intermediates and Low-Bandgap Polymers by Electropolymerization of Thienoazaborines,
O. Lukoyanova, M. Lepeltier, M. Laferrière, D. F. Perepichka, Macromolecules 2011, 44, 4729–4734.
New azaborine-thiophene heteroacenes,
M. Lepeltier, O. Lukoyanova, A. Jacobson, D.F. Perepichka, Chem. Comm. 2010, 7007-7009
New Highly Emissive Thienylene-Vinylene Oligomers and Co-Polymers for Organic Electronics,
S. Jeeva, O. Lukoyanova, A. Karas, A. Dadvand, F. Rosei, D.F. Perepichka, Adv. Funct. Mater. 2010, 20, 1661-1669
Synthesis, Polymerization and Unusual Properties of New Star-Shaped Thiophene Oligomers,
T. Taerum, O. Lukoyanova, R. Wylie, D. F. Perepichka, Org. Lett. 2009, 11, 3230-3233
Towards crystal engineering of solid state polymerization in dibromothiophenes,
M. Lepeltier, J. Hiltz, T. Lockwood, F. Belanger-Gariepy, D. F. Perepichka, J. Mater. Chem. 2009, 19, 5167-5174
A New Structural Motif in Thienoacene Semiconductors: Synthesis, Structure and Properties of Tetrathienoanthracene Isomers,
J. L. Brusso, O. Hirst, A. Dadvand, S. Ganesan, F. Cicoira, C. M. Robertson, R. T. Oakley, F. Rosei, D. F. Perepichka, Chem. Mater. 2008, 20, 2484-2494
Combining High Electron Affinity and Intramolecular Charge Transfer in Nitrofluorene - 1,3-Dithiole Push-Pull Diads,
D. F. Perepichka, I. F. Perepichka, O. Ivasenko, A J. Moore, M. R. Bryce, L. G. Kuzmina, A.S. Batsanov, N.I. Sokolov, Chem. Eur. J. 2008, 14, 2757-2770
A new simple synthesis of poly(thiophene-methine)s,
Md. B. Zaman, D. F. Perepichka, Chem. Commun. 2005, 4187-4189
Organic semiconductor devices
Organic semiconductors are the key materials enabling a variety of (opto)electronic applications, such as organic light-emitting diodes (OLED), organic field-effect transistors (OFET), organic photovoltaics (OPV) and sensors. Apart of many “engineering” advantages of organic vs inorganic semiconductors (low-cost device fabrication, flexibility, multifunctionality, etc.), we are especially motivated by the ability for tailoring the properties of organic materials and device through modification of molecular structure.
In particular, OFET are commonly fabricated and interrogated in our lab because field-dependent electrical measurements provide a broad platform for fundamental understanding of charge transport through materials, separating the effects of charge carrier concentration, mobility and charge trap densities. A number of other devices, including light-emitting transistors (see the image below), dual-channel transistors, photovoltaic cells, and photodiodes have also been studied in the past.

Some of the recently studied fundamental questions addressed in this work include:
• What limits luminescence efficiency in high-mobility organic semiconductors?
• How the effects of molecular structure and packing (polymorphism) on the measured charge mobility can be separated?
• Can high charge mobility be achieved in large band-gap organic semiconductors?
• How active dual-channel (ambipolar) semiconducting materials (eg, bulk heterojunction) be fabricated via H-bonding self-assembly?
Selected Publications
-
A Wide Bandgap Naphthalene Semiconductor for Thin-Film Transistors,
L. Yan, F. Popescu, M. R. Rao, H. Meng, D. F. Perepichka, Adv. Electron. Mater. 2017, 3, 1600556
Flexible Asymmetric Supercapacitors via Spray-Coating of a New Electrochromic Donor-Acceptor Polymer,
Y. Guo, W. Li, H. Yu, D. F. Perepichka, H. Meng, Adv. Ener. Mater. 2017, 7, 1601623
Effects of Heteroatoms on the Charge Mobility of Anthracene Derivatives,
L. Yan, Y. Zhao, H. Yu, Z. Hu, Y. He, O. Goto, C. Yan, T. Chen, R. Chen, Y.-L. Loo, D. F. Perepichka, H. Meng, W. Huang, J. Mater. Chem. C 2016, 4, 3517–3522
Polymorphism in New Thienothiophene-Thiazolothiazole Organic Semiconductors,
J. A. Schneider, H. T. Black, H. Lin, D. F. Perepichka, ChemPhysChem 2015, 6, 1173-1178
Crystal Engineering of Dual Channel p/n Organic Semiconductors by Complementary Hydrogen Bonding,
H. T. Black, D. F. Perepichka, Angew. Chem. Int. Ed. 2014, 53, 2138–2141
Dithienonaphthothiadiazole Semiconductors: Synthesis, Properties, and Application to Ambipolar Field Effect Transistors,
Q. Shuai, H. T. Black, A. Dadvand, D. F. Perepichka, J. Mater. Chem. C. 2014, 2, 3972–3979
Oligofuran-containing molecules for organic electronics,
O. Gidron, A. Dadvand, W.-H. Sun, I. Chung, L. J. W. Shimon, M. Bendikov, D. F. Perepichka, J. Mater. Chem. C 2013, 1, 4358–4367
1,5-, 2,6- and 9,10-Distyrylanthracenes as luminescent organic semiconductors,
A. Dadvand, A. G. Moiseev, W.-H. Sun, F. Bélanger-Gariépy, F. Rosei, H. Meng, D. F. Perepichka, J. Mater. Chem. C 2013, 1, 2817-2825
Perfluoroalkyl-substitution versus electron-deficient building blocks in design of oligothiophene semiconductors,
H. T. Black, A. Dadvand, S. Liu, V. S. Ashby, D. F. Perepichka, J. Mater. Chem. C 2013, 1, 260–267
Maximizing field-effect mobility and solid-state luminescence in organic semiconductors,
A. Dadvand, A.G. Moiseev, K. Sawabe, W.-H. Sun, B. Djukic, I. Chung, T. Takenobu, F. Rosei, D.F. Perepichka, Angew. Chem.Int. Ed. 2012, 51, 3837-3841
Towards "green" Electronic Materials. α-Oligofurans as Semiconductors,
O. Gidron, A. Dadvand, Y.Sheynin, M. Bendikov, D.F. Perepichka, Chem. Commun. 2011, 1976-1979
New Highly Emissive Thienylene-Vinylene Oligomers and Co-Polymers for Organic Electronics,
S. Jeeva, O. Lukoyanova, A. Karapanayiotis, A. Dadvand, F. Rosei, D.F. Perepichka, Adv. Funct. Mater. 2010, 20, 20, 1661-1669.
Quasi Temperature Independent Charge Carrier Mobility in Hexagonal Columnar Mesophases of H-Bounded Benzotristhiophene Derivative,
A. Demenev, S. H. Eichhorn, T. Taerum, D. F. Perepichka, S. Patwardhan, F. C. Grozema, L. D. A. Siebbeles, Chem. Mater. 2010, 22, 1420-1428
Heterocirculenes as a new class of organic semiconductors,
A. Dadvand, F. Cicoira, K. Yu. Chernichenko, E. S. Balenkova, R. M. Osuna, F. Rosei, V. G. Nenajdenko, D. F. Perepichka, Chem. Commun. 2008, 5354-5356
Environmentally stable organic light emitting field effect transistors based on 2-(4-pentylstyryl)tetracene,
F. Cicoira, C. Santato, A. Dadvand, C. Harnagea, A. Pignolet, P. Bellutti, Z. Xiang, F. Rosei, H. Meng, D.F. Perepichka, J. Mater. Chem. 2008, 18, 158-161
Supramolecular Control of Organic Electronic Materials
Nature uses weak non-covalent interactions to build complex biomolecules and whole organisms through a process of self-assembly. We would like to employ such self-assembly properties of organic matter for bottom-up construction of complex (opto)electronic materials and devices based on pi-conjugated molecules.
We design and synthesize (hetero)aromatic molecules with weakly binding functionalities (hydrogen bonding, halogen bonding, etc) which can be used as “nanoscopic bricks ” to build semiconducting films and crystals with a precise control of molecular packing, orientation, periodicity, with a goal to gain control over molecular properties in the solid state. We use high-resolution AFM and STM (see image below) to directly visualize how molecular associations are formed in the thin films. Combining this data with X-ray crystallography, DFT calculations, and electrical measurements allows us to develop a fundamental understanding of supramolecular structure – electronic properties relationships.
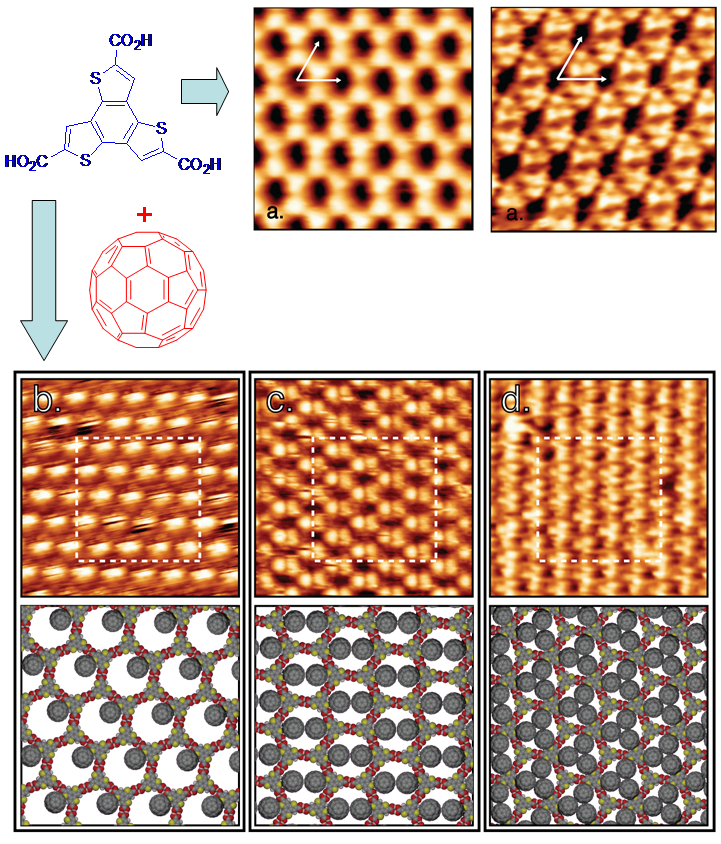
Selected Publications
-
H-bonding Control of Supramolecular Ordering of Diketopyrrolopyrroles,
C. Fu, P. J. Beldon, D. F. Perepichka, Chem. Mater. 2017, 29, 2979–2987
Patchy Nanofibers from the Thin Film Self-Assembly of a Conjugated Diblock Copolymer
E. Kynaston, Y. Fang, J. G. Manion, N. K. Obhi, J. Y. Howe, D. F. Perepichka, D. S. Seferos, Angew. Chem. Int. Ed. 2017, 56, 6152–6156
Controlling C60 growth through dipole-induced band alignment at self-assembled monolayer interface,
M. A. Mezour, O. Voznyy, E. Sargent, R. B. Lennox, D. F. Perepichka, Chem. Mater. 2016, 28, 8322–8239
Synthesis of Macrocyclic Poly(3-hexylthiophene) and Poly(3-heptylselenophene) by Alkyne Homocoupling,
G. R. McKeown, Y. Fang, N. K. Obhi, J. G. Manion, D. F. Perepichka, D. S. Seferos, ACS Macro Lett. 2016, 5, 1075–1079
Complementary Hydrogen Bonding Modulates Electronic Properties and Controls Self-Assembly of Donor/Acceptor Semiconductors,
H. T. Black, N. Yee, Y. Zems, D. F. Perepichka, Chem. Eur. J. 2016, 22, 17251–17261
Supramolecular ordering of difuryldiketopyrrolopyrrole: the effect of alkyl chains and inter-ring twisting,
C. Fu, F. Belanger-Gariepy, D. F. Perepichka, Cryst. Eng. Comm. 2016, 18, 4285–4289
Unravelling the self-assembly of hydrogen bonded semiconductors in 2D and 3D,
C. Fu, H.-P. Lin, J. L. Macleod, A. Krayev, F. Rosei, D. F. Perepichka, Chem. Mater. 2016, 28, 951–961
Substrate, molecular structure and solvent effects in 2D self-assembly via hydrogen and halogen bonding,
R. Gatti, J. M. Macleod, J. A. Lipton-Duffin, A. Moiseev, D. F. Perepichka, F. Rosei, J. Phys. Chem. C 2014, 118, 25505-25516
Supramolecuar control of organic p/n-heterojunctions by complementary hydrogen bonding,
H.T. Black, H. Lin, F. Bélanger-Gariépy, D.F. Perepichka, Faraday Discuss. 2014, 174, 297-312
Crystal Engineering of Dual Channel p/n Organic Semiconductors by Complementary Hydrogen Bonding,
H. T. Black, D. F. Perepichka, Angew. Chem. Int. Ed. 2014, 53, 2138–2141
2D self-assembly of fused oligothiophenes: molecular control of morphology,
C. Fu, F. Rosei, D. F. Perepichka, ACS Nano 2012, 6, 7973–7980
Halogen bonds in 2D supramolecular self-assembly of organic semiconductors,
R. Gutzler, C. Fu, A. Dadvand, Y. Hua, J. MacLeod, F. Rosei, D. F. Perepichka, Nanoscale 2012, 4, 5965–5971
Halogen bonds as stabilizing interactions in a chiral self-assembled molecular monolayer,
R. Gutzler, O. Ivasenko, C. Fu, J. L. Brusso, F. Rosei*, D. F. Perepichka*, Chem. Commun. 2011, 47, 9453–9455
Supramolecular ordering in oligothiophene-fullerene monolayers studied by STM,
J.M. MacLeod, O. Ivasenko, C. Fu, T. Taerum, F. Rosei, D.F. Perepichka, J.Am. Chem. Soc. 2009, 131, 16844-16850.
Supramolecular assembly of heterocirculenes in 2D and 3D,
O. Ivasenko, J. M. MacLeod, K. Yu. Chernichenko, E. Balenkova, R. V. Shpachenko, V. G. Nenajdenko, F. Rosei, D. F. Perepichka, Chem. Commun., 2009, 1192-1194.
Molecular assembly of rubrene on a metal/metal oxide nanotemplate,
F. Cicoira, J. A. Miwa, D. F. Perepichka, F. Rosei, J. Phys. Chem. A 2007, 111, 12674-12678.
Two-dimensional Conjugated Polymers
Discovery of special electronic properties of graphene is an event of a century in solid state physics. Unfortunately, zero band-gap of graphene severely limits applications because its electrical conductivity cannot be turned on and off by external stimuli (as in transistors). Several methods of chemical modifications of graphene are known, but all of them necessarily destroy the electronic properties by saturating its double bonds. Our approach is based on design of “organic graphenes”, i.e. two-dimensional pi-conjugated polymers from molecular building blocks such that the optoelectronic properties of the resulting 2D materials can be finely tuned by the choice of the monomer.
Apart of synthesis of the tailored building blocks, the major activity is devoted to devising methods of their polymerization, which can proceed in an ordered 2D fashion (as oppose to random 3D growth). The two ideas that were so far brought to fruition are:
- (I) Surface-templated polymerization where the reactive monomers are assembled on atomically flat (usually catalytic) surfaces and polymerized by annealing. This approach allows using STM to directly observed the polymerization process (see the figure), and directly delivers a single monolayer of the 2D polymer. This work is carried out in collaboration with the group of Prof. Rosei (INRS) and other surface physicists.
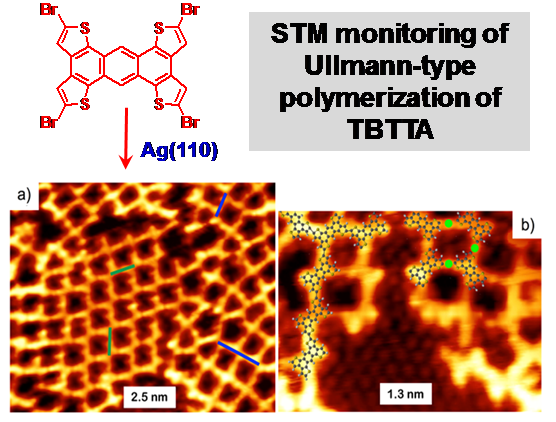
- (ii) Dynamic covalent polymerization. This approach relies on application of reversible reactions to link the monomers in a “dynamic” fashion where the (less stable) defective connections can be “undone” and reconnected, until an ordered crystalline stack of 2D polymers is produced (see example below).
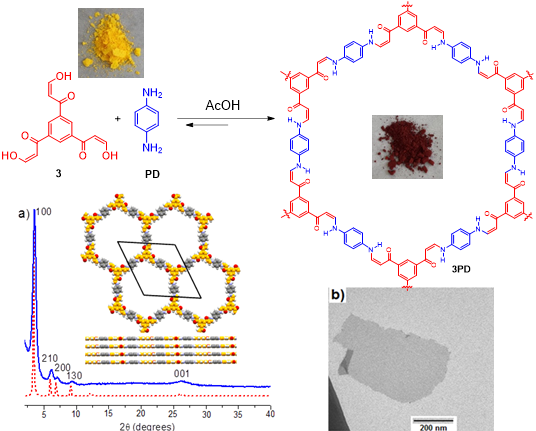
Selected Publications
2D Poly(arylene vinylene) Covalent Organic Frameworks via Aldol Condensation of Trimethyltriazine,
T. Jadhav, F. Yuan, W. Patterson, C.-H. Liu, E. Hamzehpoor, D. F. Perepichka, Angew. Chem. Int. Ed.. 2019, xx, xxxx–xxxx
Conjugated Covalent Organic Frameworks via Michael Addition‒Elimination,
M. R. Rao, Y. Fang, S. De Feyter, D. F. Perepichka, J. Am. Chem. Soc. 2017, 139, 2421–2427
Mechanistic Picture and Kinetic Analysis of Surface-Confined Ullmann Polymerization,
M. Di Giovannantonio, M. Tomellini, J. Lipton-Duffin, G. Galeotti, M. Ebrahimi, A. Cossaro, A. Verdini, N. Kharche, V. Meunier, G. Vasseur, Y. Fagot-Revurat, D. F. Perepichka, F. Rosei, G. Contini, J. Am. Chem. Soc. 2016, 138, 16696–16702
Quasi one-Dimensional Band Dispersion and Surface Metallization in Long Range Ordered Polymeric Wires,
G. Vasseur, Y. Fagot-Revurat, M. Sicot, B. Kierren, D. Malterre, L. Cardenas, G. Galeotti, J. Lipton-Duffin, F. Rosei, M. Di Giovannantonio, G. Contini, P. Lefevre, F. Bertran, V. Meunier, L. Liang, D.F. Perepichka, Nat. Comm. 2016, 7, 10235
Ullmann-Type Coupling of Brominated Tetrathienoanthracene on Crystalline Copper and Silver,
R. Gutzler, L. Cardenas, J. Lipton-Duffin, M. El Garah, L. E. Dinca, C. E. Szakacs, C. Fu, M. Gallagher, M. Vondracek, M. Rybachuk, D. F. Perepichka, F. Rosei, Nanoscale 2014, 6, 2660–2668
pi-Electron conjugation in two dimensions,
R. Gutzler, D. F. Perepichka, J. Am. Chem. Soc. 2013, 135, 16585–16594
Synthesis and electronic structure of a 2D p-Conjugated Polythiophene,
L. Cardenas, R. Gutzler, J. Lipton-Duffin, C. Fu, J.L. Brusso, L.E. Dinca, M. Vondráček, Y. Fagot-Revurat, D. Malterre, F. Rosei, D.F. Perepichka, Chem. Sci. 2013, 4, 3263–3268
Insight into Organometallic Intermediate and its Evolution to Covalent Bonding in Surface-Confined Ullmann Polymerization,
M. Di Giovannantonio, M. El-Garah, J. Lipton-Duffin, V. Meunier, L. Cardenas, Y. Fagot-Revurat, A. Cossaro, A. Verdini, D. F. Perepichka, F. Rosei, G. Contini, ACS Nano 2013, 7, 8190–8198
Step-by-step growth of aligned polythiophene wires by surface-confined oligomerization,
J.A. Lipton-Duffin, J.A. Miwa, M. Kondratenko, F. Cicoira, B.G. Sumpter, V. Meunier, D.F. Perepichka, F. Rosei, Proc. Nat. Acad. Sci. USA. 2010, 107, 11200-11204
Extending Polymer Conjugation in the Second Dimension,
D. F. Perepichka, F. Rosei, Science 2009, 323, 216-217
Synthesis of polyphenylene molecular wires by surface confined polymerization,
J. A. Lipton-Duffin, O. Ivasenko, D. F. Perepichka, F. Rosei, Small 2009, 5, 592-597
Nanochemistry
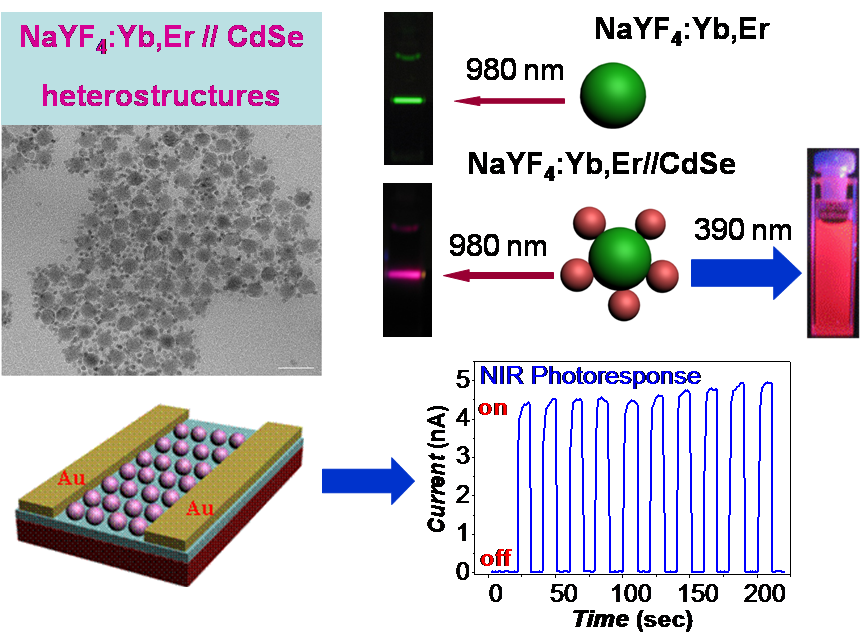
While organic materials are the focus of majority of our projects, occasional brave students and post-docs take the leap out of our comfort zone, exploring the optoelectronic properties and self-assembly of inorganic materials. In the past, we have worked on design of asymmetric functionalization of single-wall carbon nanotubes; light up-conversion in nanostructured semiconducting films consisting of co-assembled quantum dots and Er-doped lanthanide nanoparticles (see image below). More recently, we have explored the possibility of using molecular network to template the assembly of gold nanoparticles in periodically structured 2D crystals.
Selected Publications
Lanthanide Ion Doped Upconverting Nanoparticles: Synthesis, Structure and Properties,
C. Yan, H. Zhao, D. F. Perepichka, F. Rosei, Small 2016, 12, 3888–3907
Directing the Assembly of Gold Nanoparticles with Two-Dimensional Molecular Networks”,
M. A. Mezour, I. I. Perepichka, J. Zhu, R. B. Lennox, D. F. Perepichka, “ACS Nano 2014, 8, 2214–2222
High thermal stability of block-copolymer capped Au and Cu nanoparticles,
I. I. Perepichka, M. A. Mezour, D. F. Perepichka, R. B. Lennox, Chem. Commun. 2014, 50, 11919–11921
NIR Photoresponse in New Up-Converting CdSe/NaYF4:Yb,Er Nano-Heterostructures,
C. Yan, A. Dadvand, F. Rosei, D.F. Perepichka, J. Am. Chem. Soc. 2010, 132, 8868-8869
Multiple NaNbO3/Nb2O5 Nanotubes: A New Class of Ferromagnetic/Semiconductor Heterostructures,
C. Yan, L. Nikolova, A. Dadvand, C. Harnagea, A. Sarkissian, D.F. Perepichka, D. Xue, F. Rosei, Adv. Mater. 2010, 22, 1741-1744
Metal Nanoparticles: From "Artificial Atoms" to "Artificial Molecules" ,
D.F. Perepichka, F. Rosei, Angew. Chem. Int. Ed. 2007, 46, 6006-6008
Rectifying diodes from asymmetrically functionalized single wall carbon nanotubes,
Z.Wei, M.Kondratenko, L.H.Dao, D.F. Perepichka, J. Am. Chem. Soc. 2006, 128, 3134-3135
Silicon Nanotubes,
D.F.Perepichka, F.Rosei, Small 2006, 2, 22

