Publications
Google Scholar
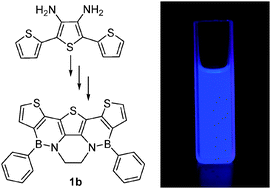
Thiele’s Fluorocarbons: Stable Diradicaloids with Efficient Vis-NIR Fluorescence from a Zwitterionic Excited State
C.-H. Liu, Z. He, C. Ruchlin, Y. Che, K. Somers, D. F. Perepichka, J. Am. Chem. Soc. 2023 (DOI: 10.1021/jacs.3c05009)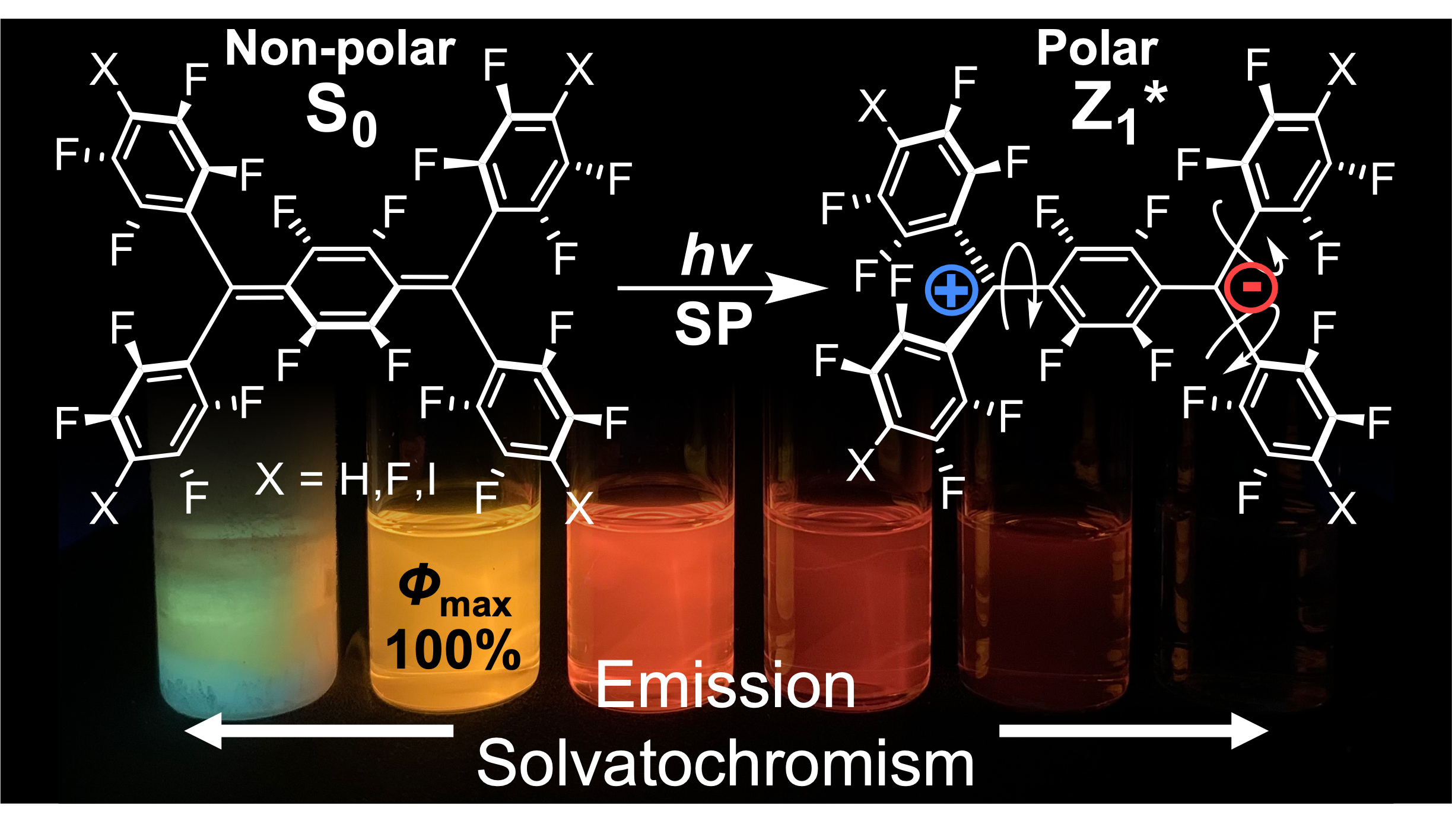
Improving Environmental and Operational Stability of Polymer Field-Effect Transistors by Doping with Tetranitrofluorenone
P. Ghamari, M. R. Niazi, D. F. Perepichka, ACS. Appl. Mater. Interfaces 2023 (DOI: 10.1021/acsami.3c01034)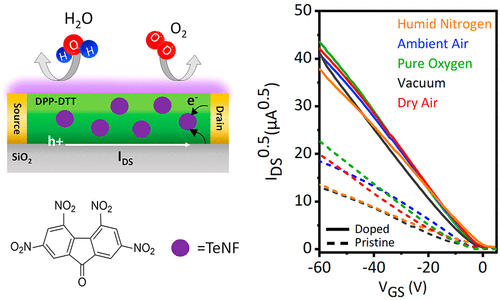
Silver nanoparticle enhanced metal-organic matrix with interface-engineering for efficient photocatalytic hydrogen evolution
Y. Liu, C.-H. Liu, T. Debnath, Y. Wang, D. Pohl, L. V. Besteiro, D. M. Meira, S. Huang, F. Yang, B. Rellinghaus, M. Chaker, D. F. Perepichka, D. Ma, Nat. Commun. 2023 (DOI: 10.1038/s41467-023-35981-8)
Rational Control of Near-Infrared Colloidal Thick-Shell Eco-Friendly Quantum Dots for Solar Energy Conversion
L. Jin, J. Liu, X. Liu, D. Benetti, G. S. Slopal, X. Tong, E. Hamzehpoor, F. Li, D. F. Perepichka, Z. M. Wang, F. Rosei, Small Methods 2023 (DOI: 10.1002/smtd.202300133)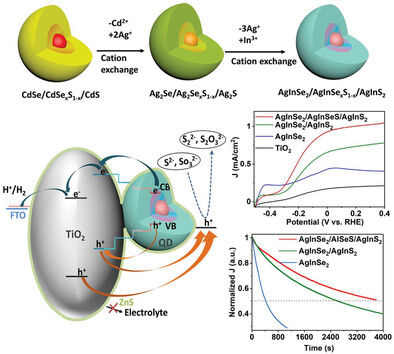
Two Dimensional Supramolecular Polymerization of DNA Amphiphiles is Driven by Sequence‐Dependent DNA‐Chromophore Interactions
M. G. Rafique, J. M. Remington, F. Clark, H. Bai, V. Toader, D. F. Perepichka, J. Li, H. F. Sleiman, Angew. Chem. Int. Ed. 2023 (DOI: 10.1002/anie.202217814)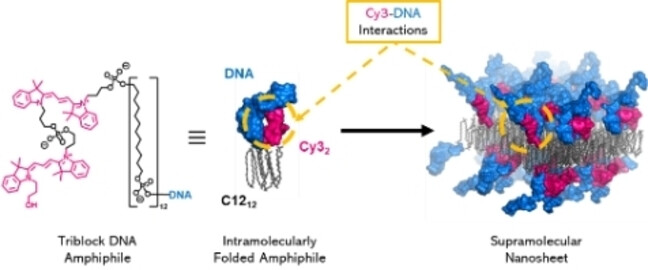
Triarylamines as catalytic donors in light-mediated electron donor–acceptor complexes
D. J. Castillo-Pazos, J. D. Lasso, E. Hamzehpoor, J. Ramos-Sánchez, J. M. Salgado, G. Cosa, D. F. Perepichka, C.-J. Li, Chem. Sci. 2023 (DOI: 10.1039/D2SC07078B)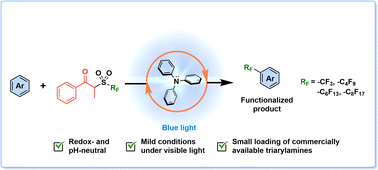
Nitrofluorene-based A–D–A electron acceptors for organic photovoltaics
Y. Che, M. R. Niazi, T. Yu, T. Maris, C.-H. Liu, D. Ma, R. Izquierdo, I. F. Perepichka, D. F. Perepichka, J. Mater. Chem. C 2023 (DOI: 10.1039/D2TC05127C)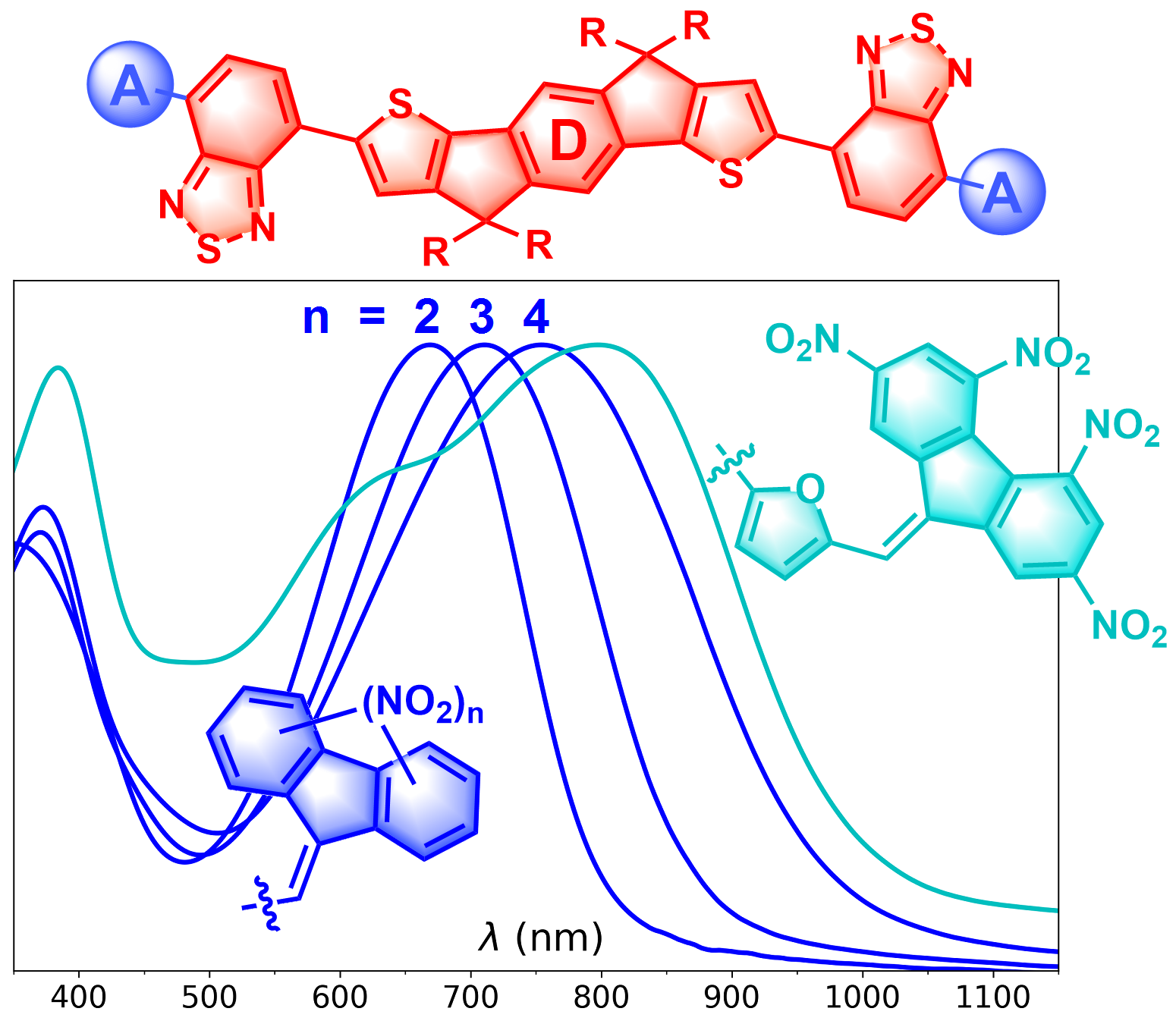
Miscibility driven morphology modulation in ternary solar cells
T. Yu, F. Tintori, Y. Zhang, W. He, E. Cieplechowicz, R. S. Bobba, P. I. Kaswekar, M. Jafari, Y. Che, Y. Wang, M. Siaj, R. Izquierdo, D. F. Perepichka, Q. Qiao, G. C. Welch, D. Ma, J. Mater. Chem. A 2023 (DOI: 10.1039/D2TA09928D)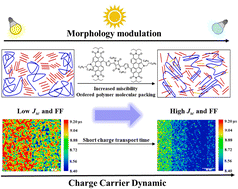
Trifluoroacetic acid prompted unexpected visible to NIR switching of ketoenamine-substituted triphenylamines
K. Laxman, Y. Che, K. A. Raj, D. F. Perepichka, M. R. Rao, J. Chem. Mater. C 2023 (DOI: 10.1039/D2TC04959G)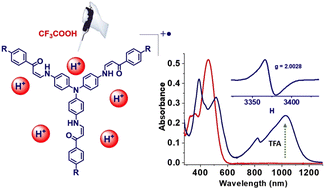
Efficient room-temperature phosphorescence of covalent organic frameworks through covalent halogen doping
E. Hamzehpoor, C. Ruchlin, Y. Tao, C.-H. Liu, H. M. Titi, D. F. Perepichka, Nat. Chem. 2022 (DOI: 10.1038/s41557-022-01070-4)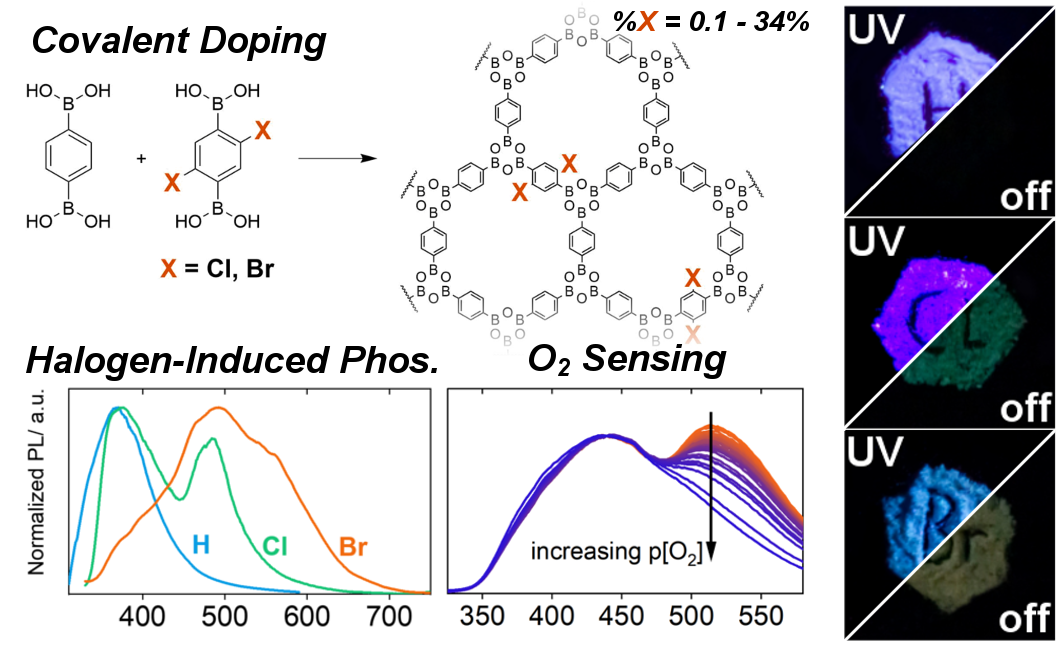
A 2D perchlorinated sp2-carbon framework
C.-H. Liu, Y. Sakai-Otsuka, P. Richardson, M. R. Niazi, E. Hamzehpoor, T. Jadhav, A. Michels-Gualteri, Y. Fang, M. Murugesu, D. F. Perepichka, Cell Rep. Phys. Sci. 2022 (DOI: 10.1016/j.xcrp.2022.100858)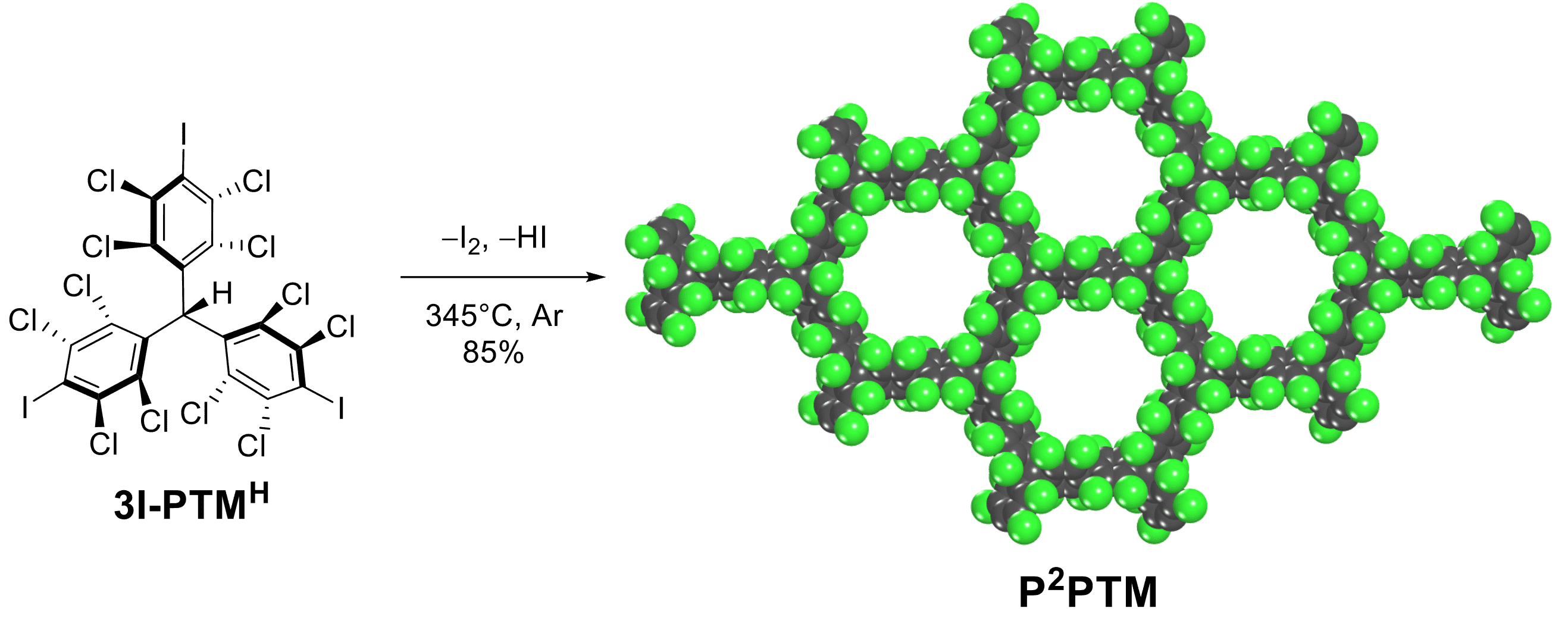
Vanishing Electronic Band Gap in Two-Dimensional Hydrogen-Bonded Organic Frameworks
C.-H. Liu, A. Wei, M.F. Cheung D. F. Perepichka, Chem. Mater. 2022 (DOI: 10.1021/acs.chemmater.2c00294)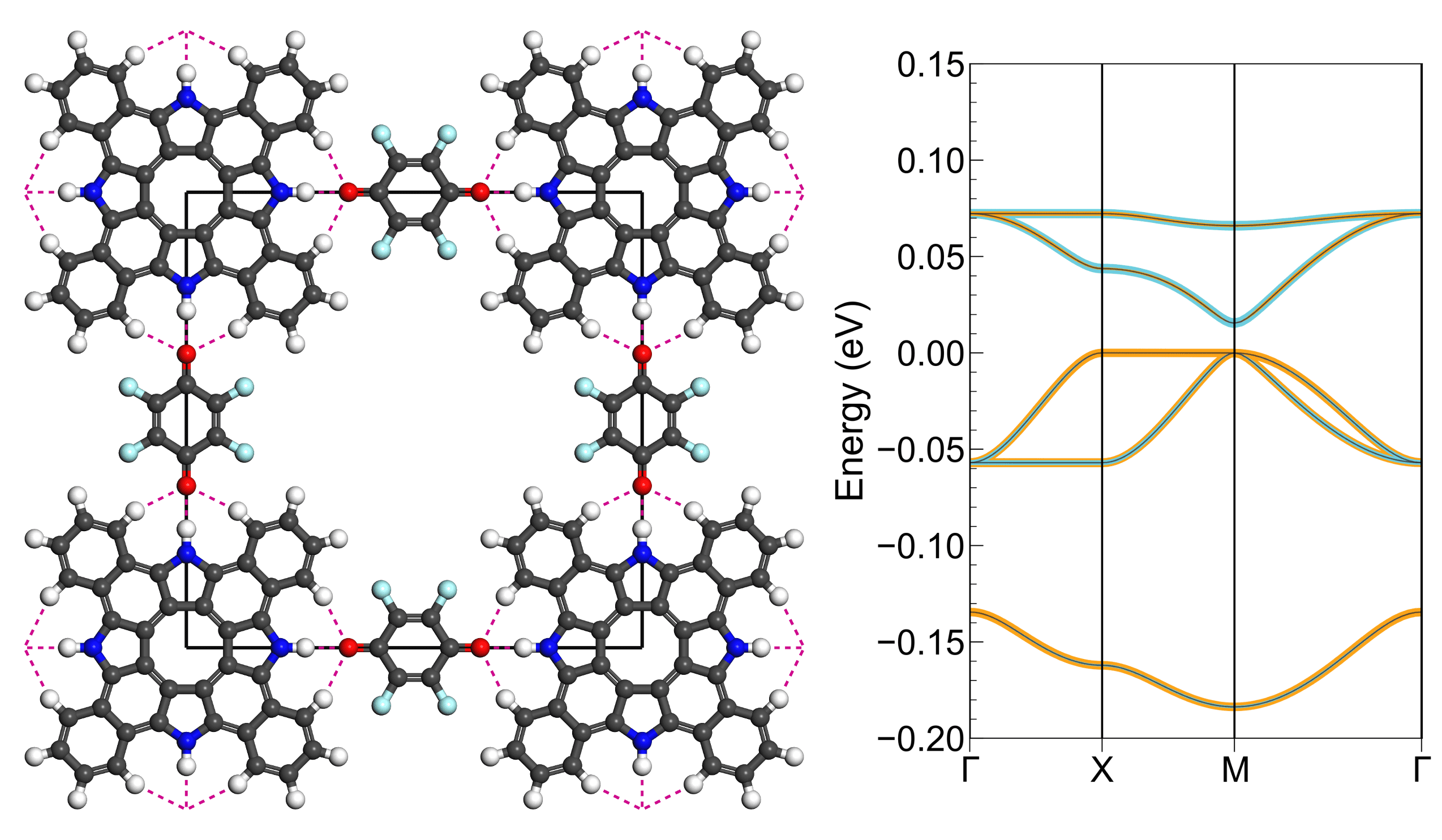
Tandem Desulfurization/C–C Coupling Reaction of Tetrathienylbenzenes on Cu(111): Synthesis of Pentacene and an Exotic Ladder Polymer
P. Ji, D. Dettmann, Y.-H. Liu, G. Berti, N. P. Genesh, D. Cui, O. MacLean, D. F. Perepichka, L. Chi, and F. Rosei, ACS Nano 2022 (DOI: 10.1021/acsnano.2c00831)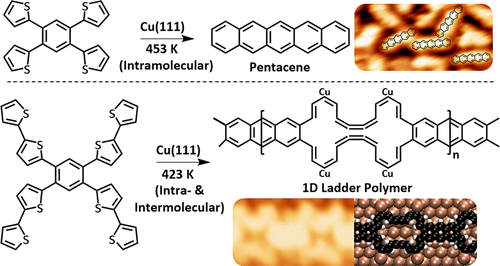
Probing the Thermodynamics of Moiré Patterns in Molecular Self-Assembly at the Liquid–Solid Interface
D. Cui, N. P. Genesh, O. MacLean, P. Ji, J. M, MacLeod, M. Ebrahimi, A. V. Lunchev, A. C. Grimsdale, D. F. Perepichka, F. Rosei, Chem. Mater. 2022 (DOI: 10.1021/acs.chemmater.2c00089)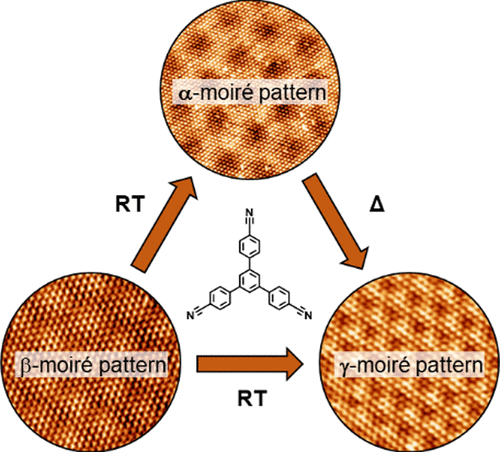
Bidirectional Phase Transformation of Supramolecular Networks Using Two Molecular Signals
D. Cui, C.-H. Liu, D. F., Perepichka, ACS Nano 2022 (DOI: 10.1021/acsnano.1c10122)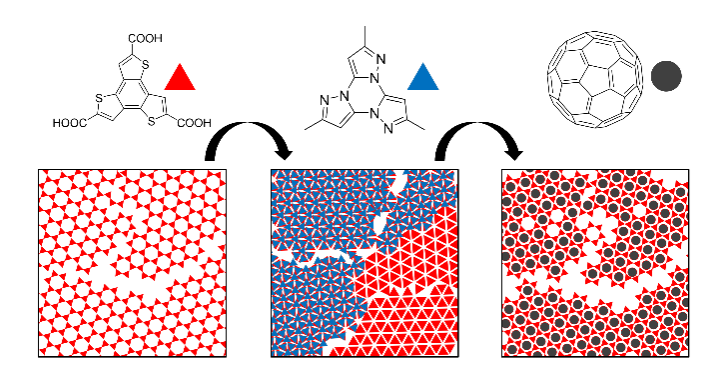
Adatoms in the Surface-Confined Ullmann Coupling of Phenyl Groups
Z. Zhang, D. F. Perepichka, R. Khaliullin, J. Phys. Chem. Lett. 2021 (DOI: 10.1021/acs.jpclett.1c02914)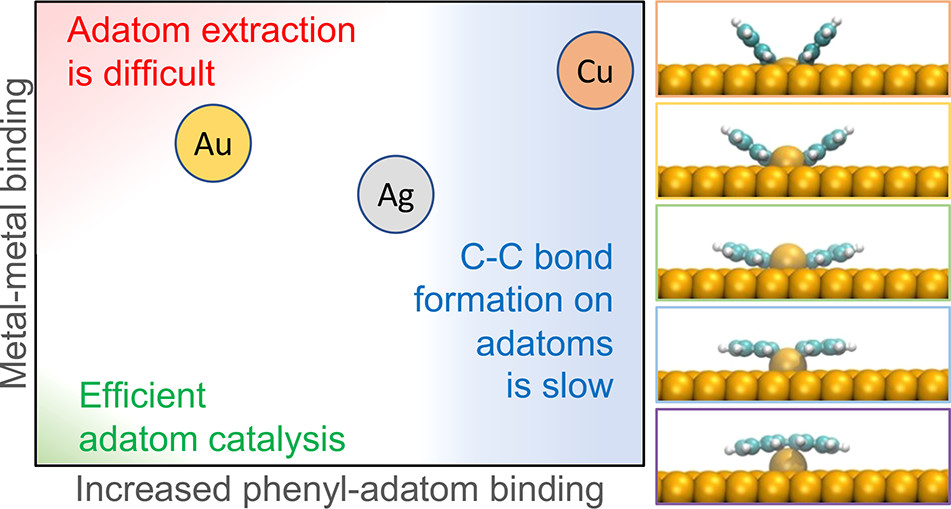
Halogen bonding vs. pi-stacking interactions in new bis(acenaphthylene)dione semiconductors
Y.-H. Liu, A. Dadvand, H. M. Titi, E. Hamzehpoor, D. F. Perepichka, CrystEngComm 2021 (DOI: 10.1039/D1CE01047F)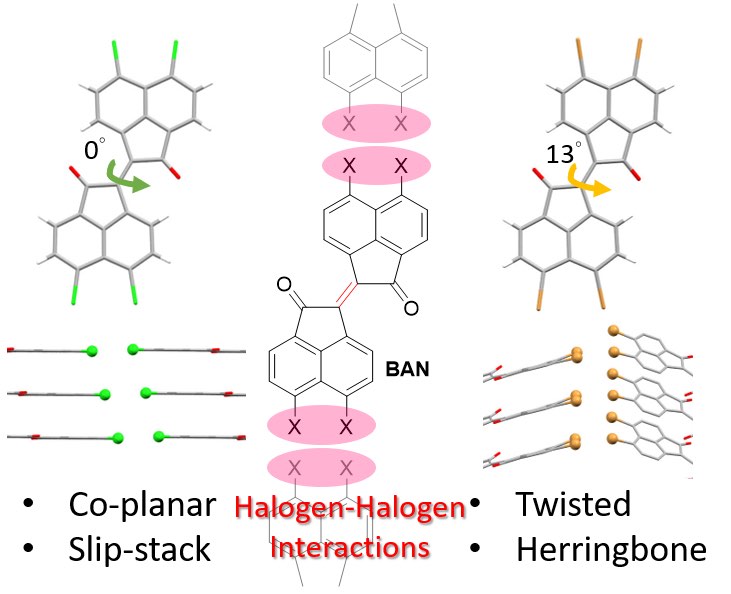
Mechanism of the Photodegradation of A-D-A Acceptors for Organic Photovoltaics
Y. Che, M. R. Niazi, R. Izquierdo, D. F. Perepichka, Angew. Chem. Int. Ed. 2021 (DOI: 10.1002/anie.202109357)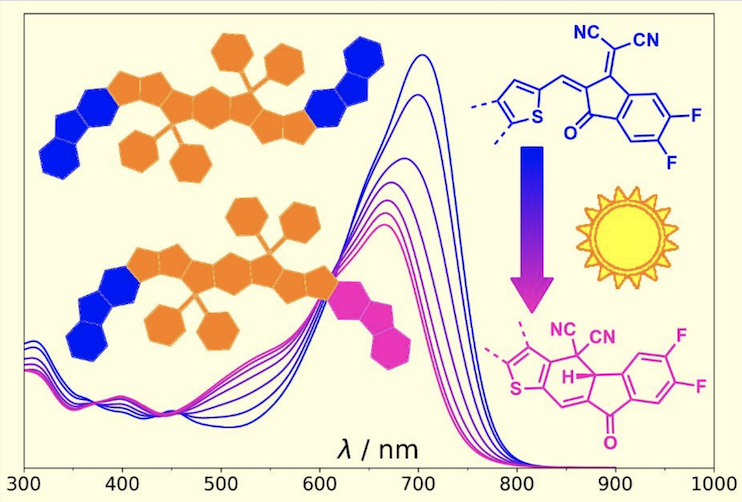
Recent advances in room temperature phosphorescence of crystalline boron containing organic compounds
H. H. Hackney, D. F. Perepichka, Aggregate 2021 (DOI: 10.1002/agt2.123)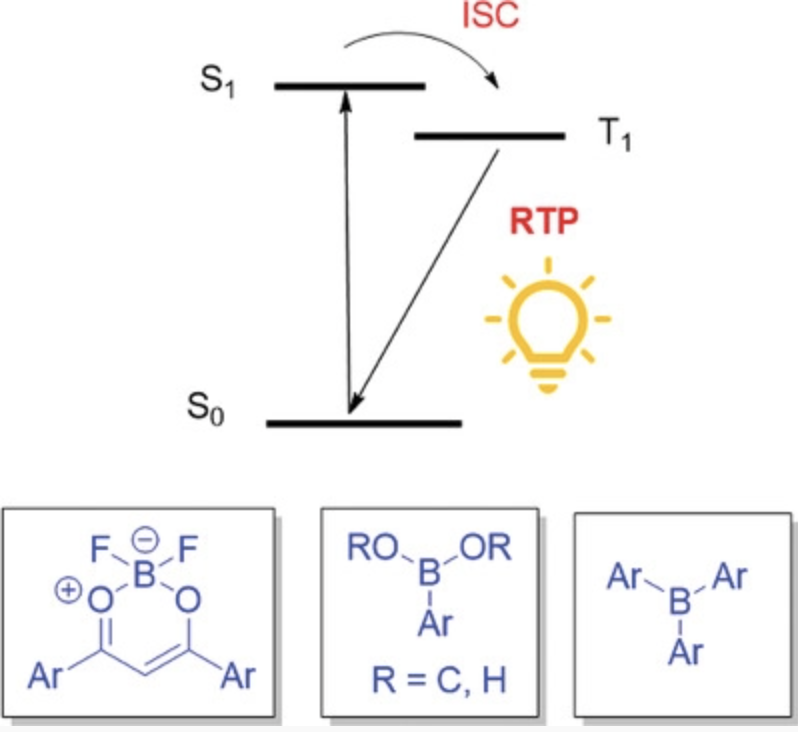
Glaser Coupling of Substituted Anthracene Diynes on a Non-metallic Surface at the Vapor-Solid Interface
Y. Fang, Z. Heydari, C.-H. Liu, N. Zhang, L. A. Cuccia, O. Ivasenko, D. F. Perepichka, Chem. Res. Chin. Uni. 2021 (DOI: 10.1007/s40242-021-1324-y). Invited contribution for special issue 'Women in Chemistry'.
Identification of Topotactic Surface-Confined Ullmann-Polymerization
D. Dettmann, G. Galeotti, O. MacLean, M. Tomellini, M. Di Giovannantonio, J. Lipton-Duffin, A. Verdini, L. Floreano, Y. Fagot-Revurat, D. F. Perepichka, F. Rosei, G. Contini, Small 2021 (DOI: 10.1002/smll.202103044)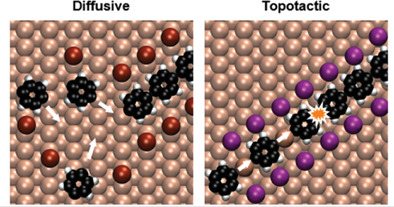
Acenaphthylene as a Building Block for pi-Electron Functional Materials
Y.-H. Liu, D. F. Perepichka, J. Mater. Chem. C 2021 (DOI: 10.1039/D1TC02826J)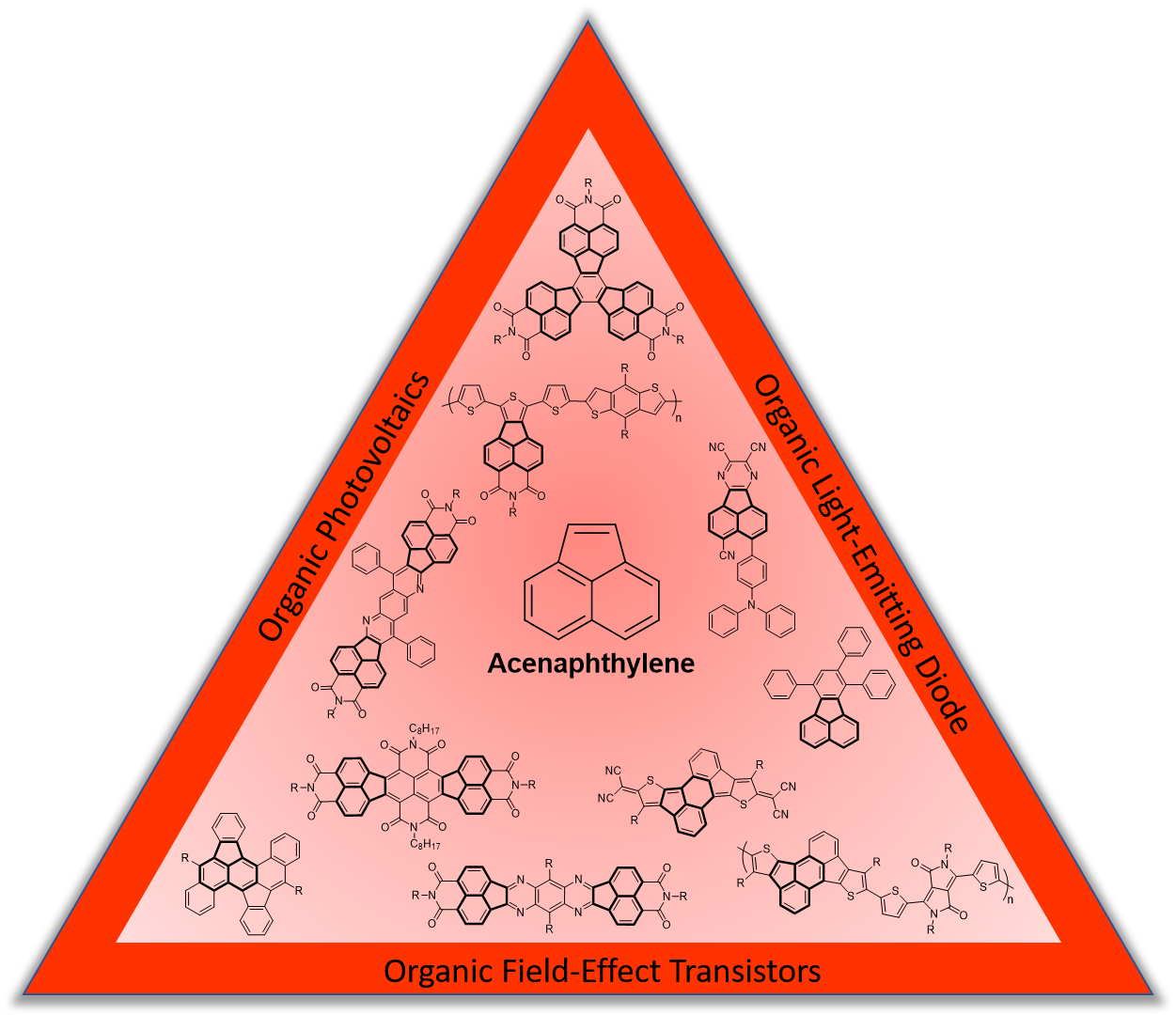
Synthesis of Boroxine and Dioxaborole Covalent Organic Frameworks via Transesterification and Metathesis of Pinacol Boronates
E. Hamzehpoor, A. Jonderian, E. McCalla, D. F. Perepichka, J. Am. Chem. Soc. 2021 (DOI: 10.1021/jacs.1c05987)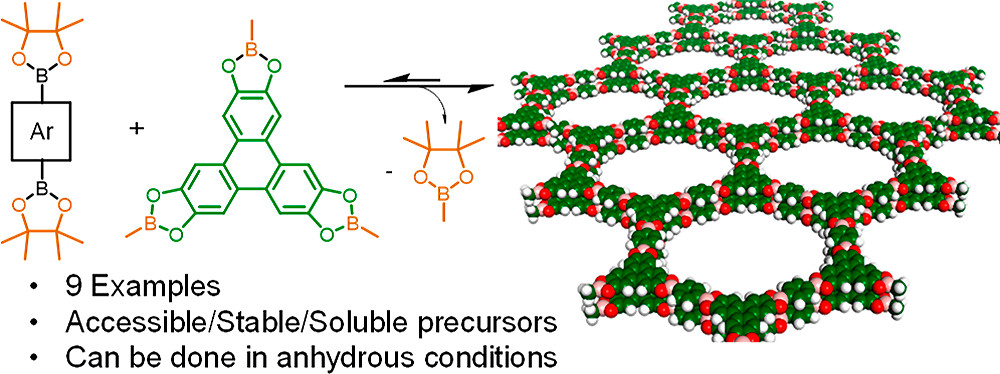
Room Temperature Phosphorescence vs Triplet–Triplet Annihilation in N-Substituted Acridone Solids
E. Hamzehpoor, C. Ruchlin, Y. Tao, J. E. Ramos-Sanchez, H. M. Titi, G. Cosa, D. F. Perepichka, J. Phys. Chem. Lett. 2021 (DOI: 10.1021/acs.jpclett.1c01552)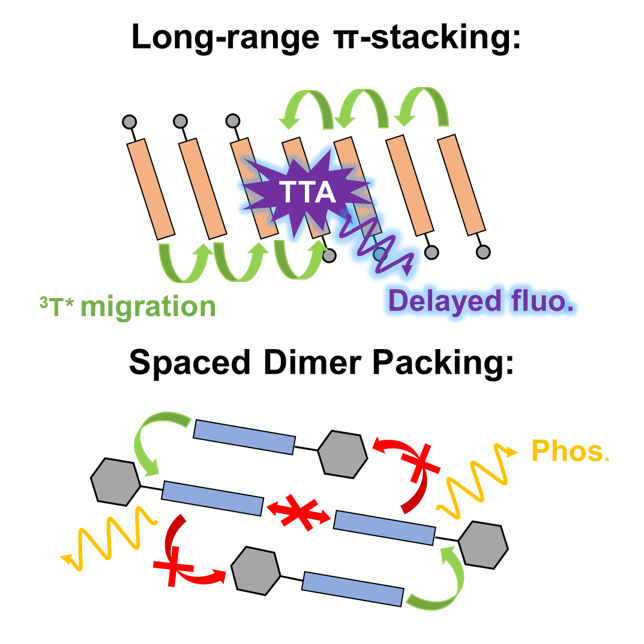
Controlling Structural and Energetic Disorder in High-Mobility Polymer Semiconductors via Doping with Nitroaromatics
P. Ghamari, M.R. Niazi, and D. F. Perepichka, Chem. Mater. 2021 (DOI: 10.1021/acs.chemmater.1c00448)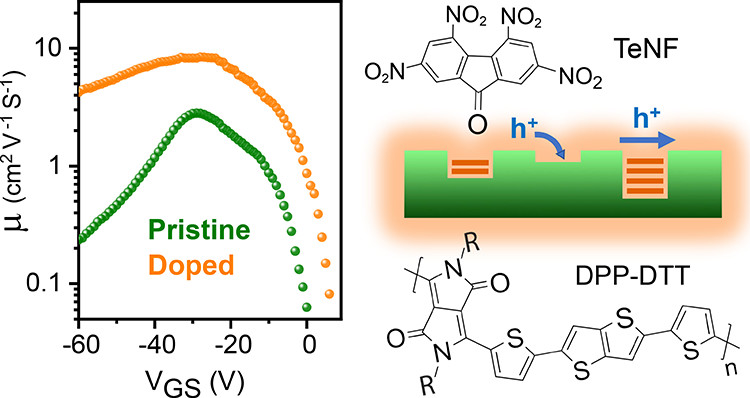
Hydrogen Bonding Versus pi-Stacking in Charge-Transfer Co-crystals
N. Yee, A. Dadvand, E. Hamzehpoor, H. M. Titi, D. F. Perepichka, Cryst. Growth Des. 2021 (DOI: 10.1021/acs.cgd.1c00309)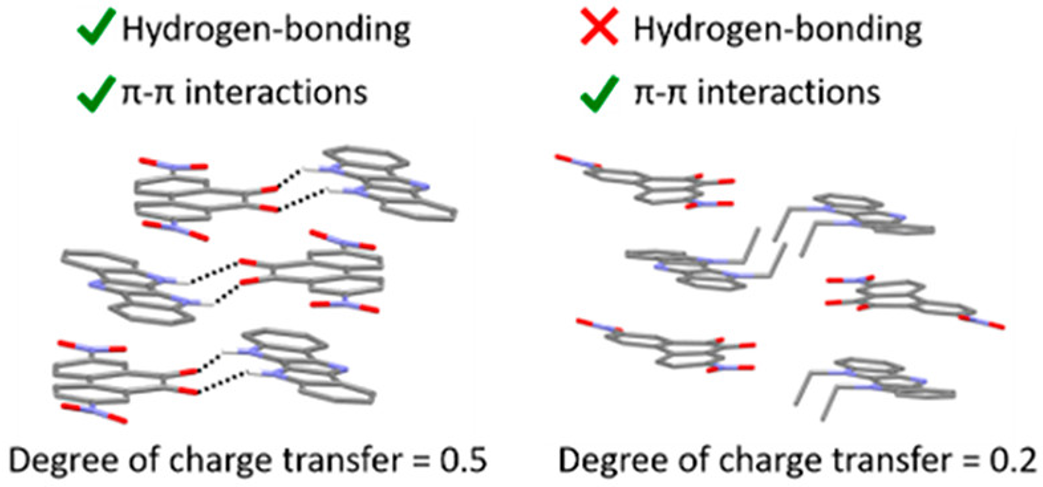
Electrically Conductive Covalent Organic Frameworks: Bridging the Fields of Organic Metals and 2D Materials
M. Souto, D. F. Perepichka, J. Mater. Chem. C 2021 (DOI: 10.1039/D1TC00750E)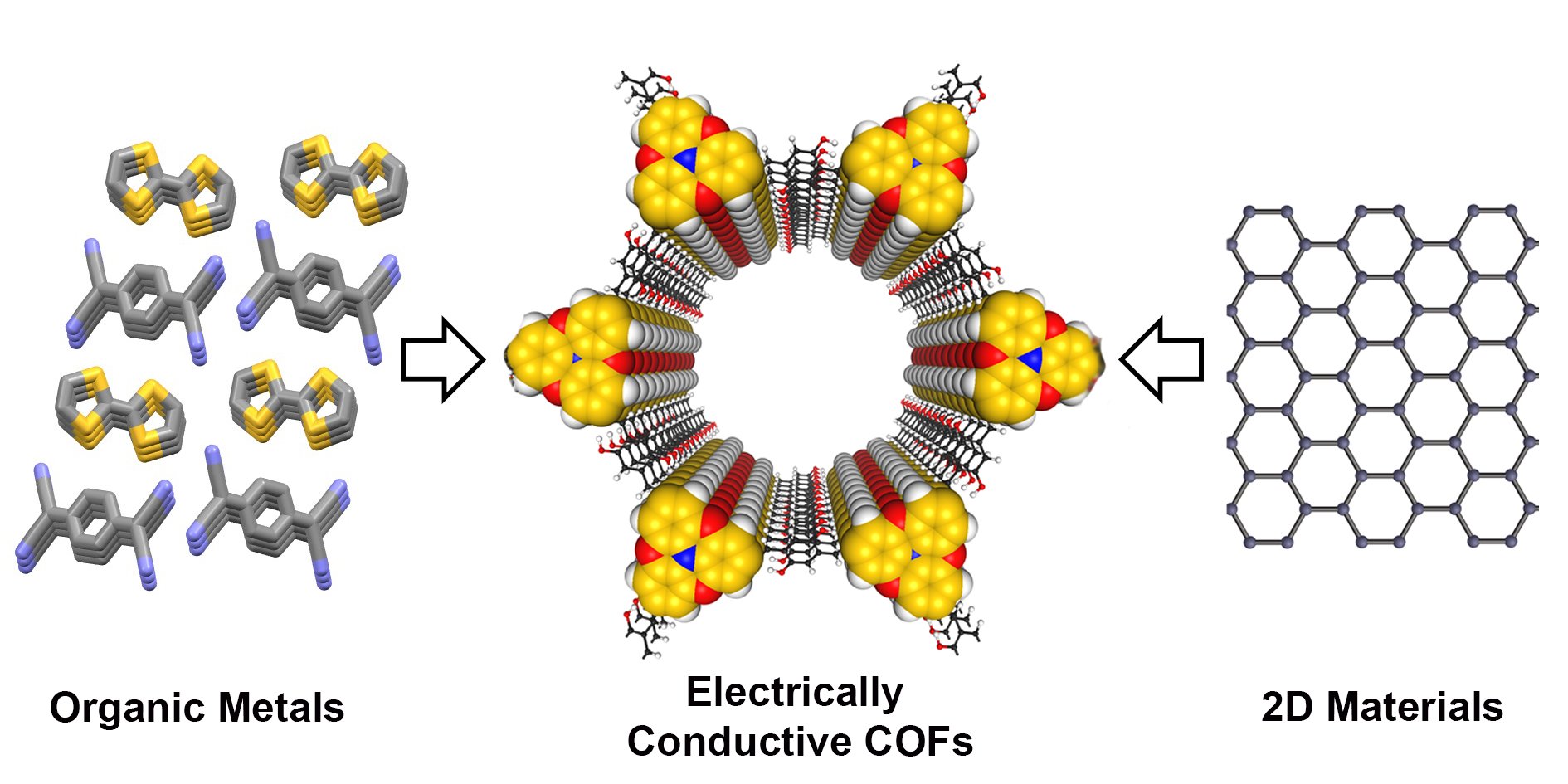
Alternating-current-Driven Color-Tunable Organic Light-Emitting Triodes
C. Zhao, M.U. Ali, J. Ji, M. Liu, A. Li, J. Bai, J. Miao, T. Wang, D. F. Perepichka, C. Yan, K.-F. C. Shen, H. Meng, Adv. Opt. Mater. 2021 (DOI: 10.1002/adom.202001655)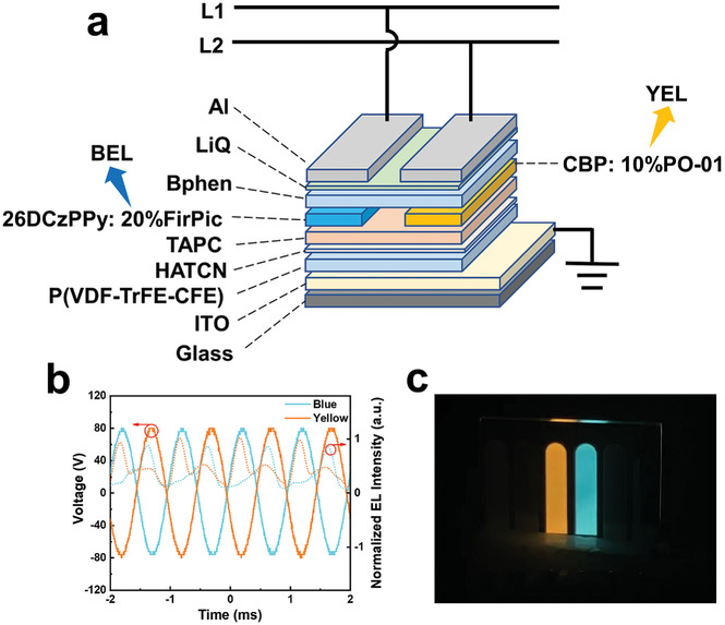
Band Gap Engineering of Donor–Acceptor Co-crystals by Complementary Two-point Hydrogen Bonding
N. Yee, A. Dadvand, D. F. Perepichka, Mater. Chem. Front. 2020 (DOI: 10.1039/D0QM00500B)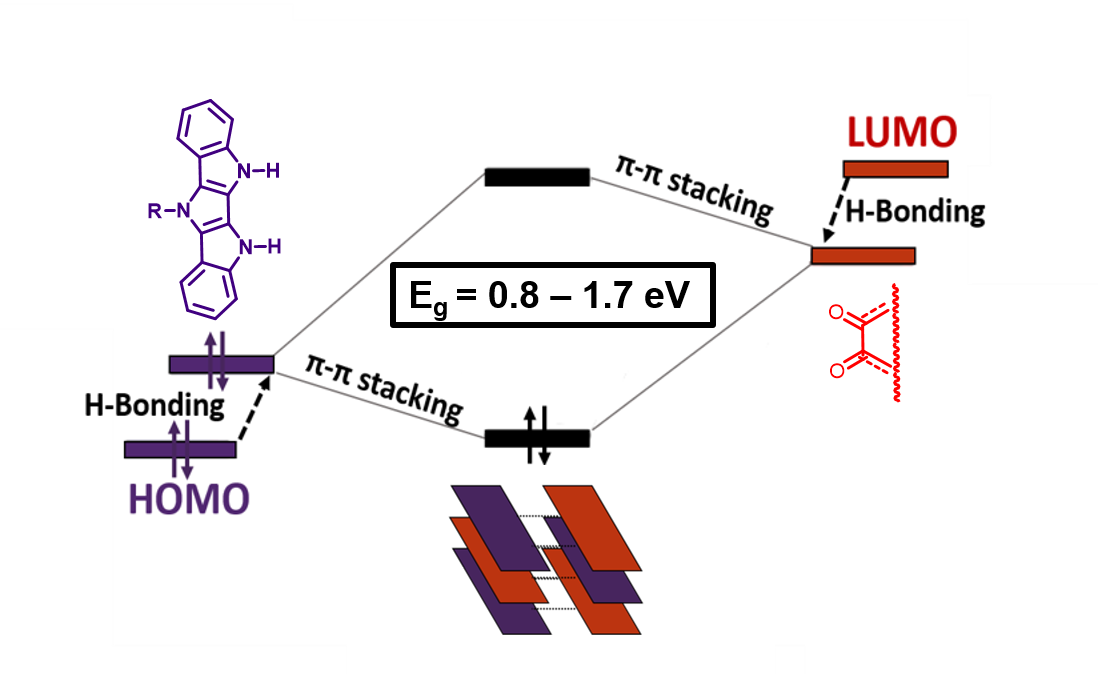
Quantifying Planarity in Design of Organic Electronic Materials
Y. Che, D. F. Perepichka, Angew. Chem. Int. Ed. 2020 (DOI: 10.1002/anie.202011521)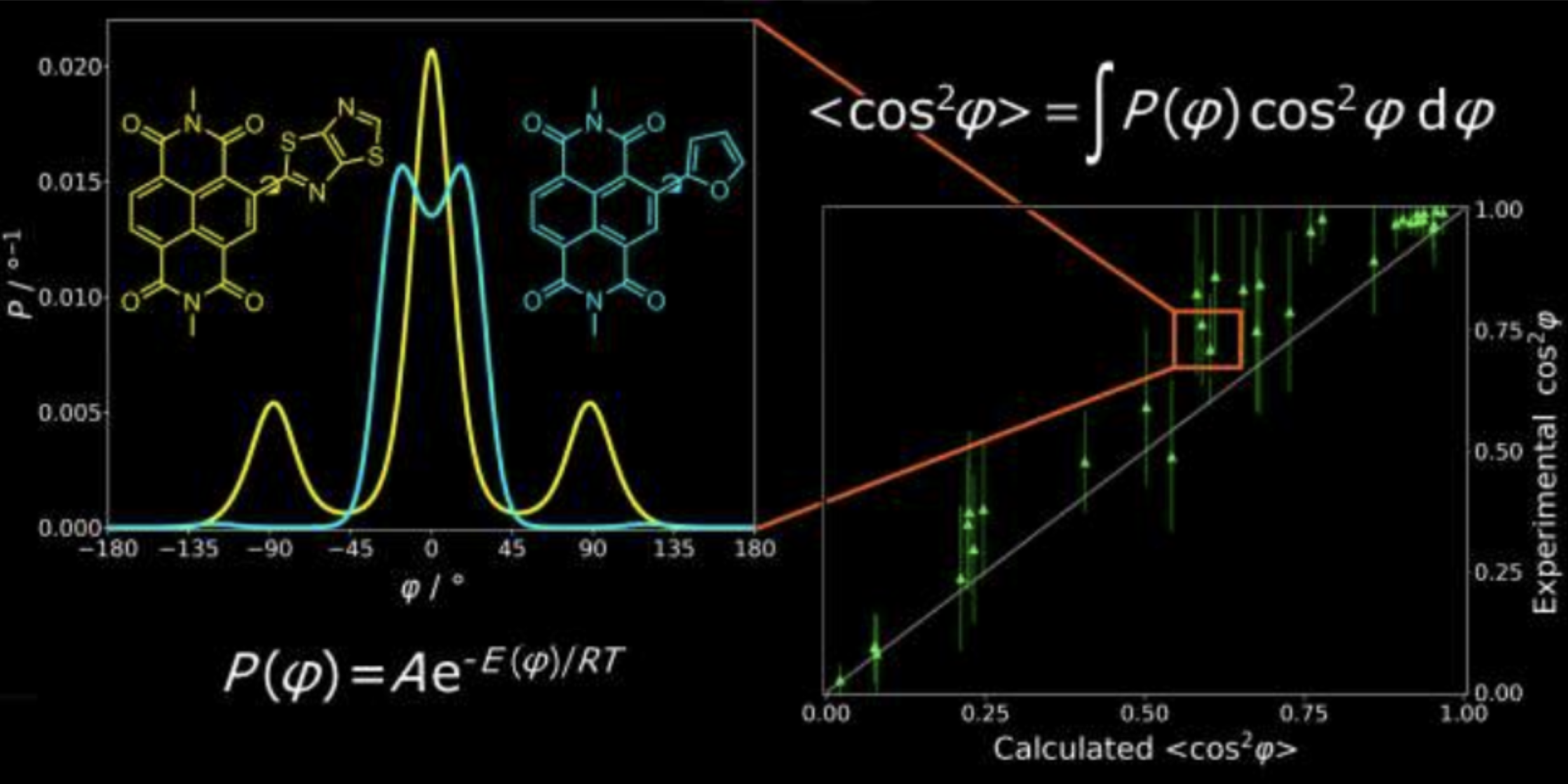
90% Quantum Yield Pure-Red Doublet Emission from Stable, Colorless, Iodinated Triphenylmethane Solid
C.-H. Liu, E. Hamzehpoor, Y. Otsuka-Sakai, T. Jadhav, D. F. Perepichka, Angew. Chem. Int. Ed. 2020 (DOI: 10.1002/anie.202009867)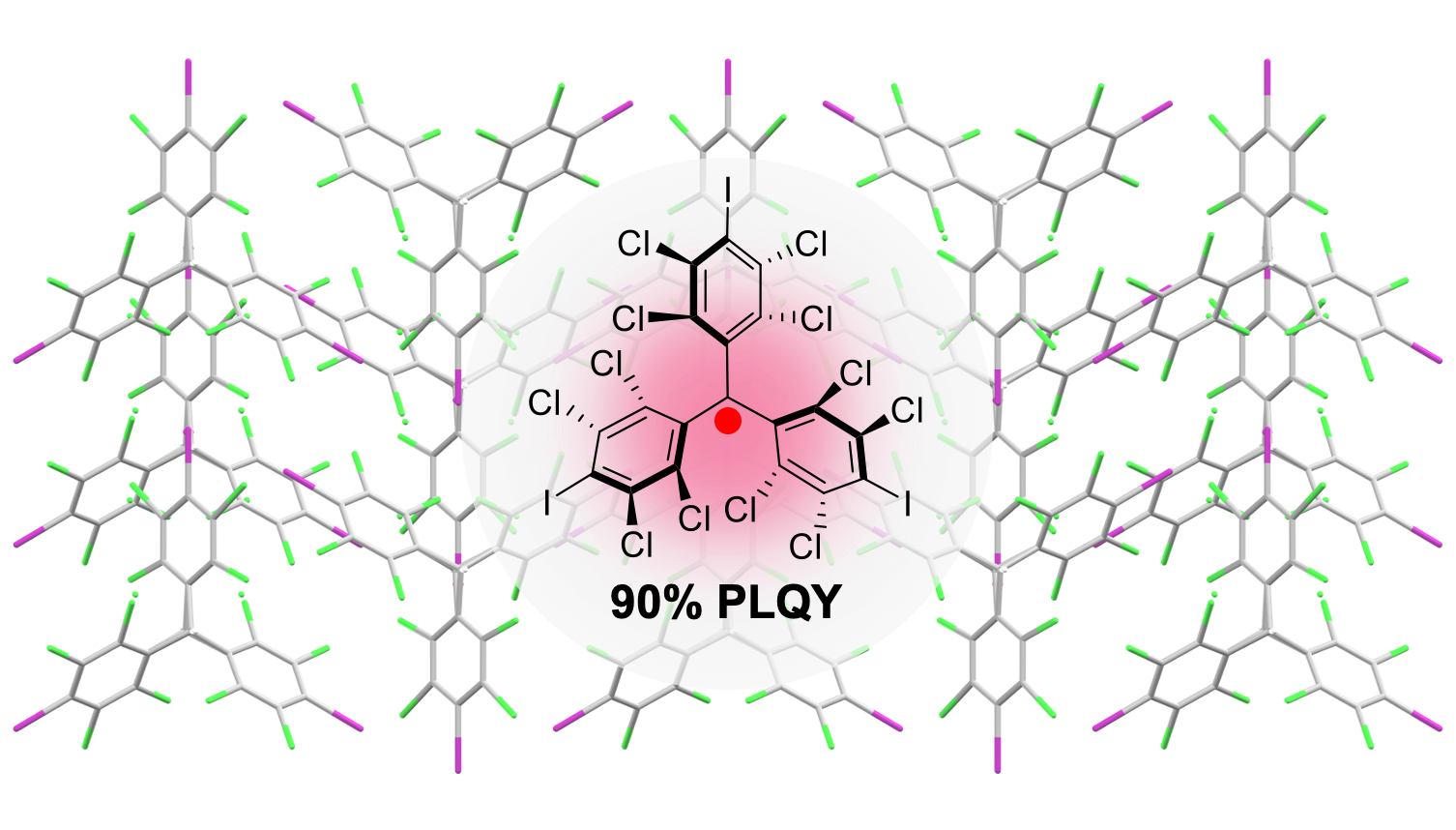
Fluorination of a Polymer Donor through the Trifluoromethyl Group for High-performance Polymer Solar Cells
C. Yao, Y. Zhu, K. Gu, J. Zhao, J. Ning, D. F. Perepichka, Y.-L. Loo, H. Meng, J. Mater. Chem. A 2020 (DOI: 10.1039/D0TA00098A)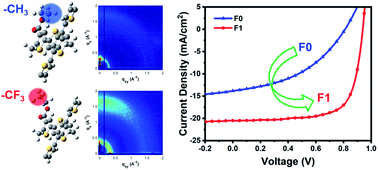
Synthesis of Mesoscale Ordered Two-dimensional pi-conjugated Polymers with Semiconducting Properties
G. Galeotti, F. De Marchi, E. Hamzehpoor, O. MacLean, M. Rajeswara Rao, Y. Chen, L. V. Besteiro, D. Dettmann, L. Ferrari, F. Frezza, P. M. Sheverdyaeva, R. Liu, A. K. Kundu, P. Moras, M. Ebrahimi, M. C. Gallagher, F. Rosei, D. F. Perepichka, G. Contini, Nat. Mater. 2020 (DOI: 10.1038/s41563-020-0682-z) Corresponding news and views article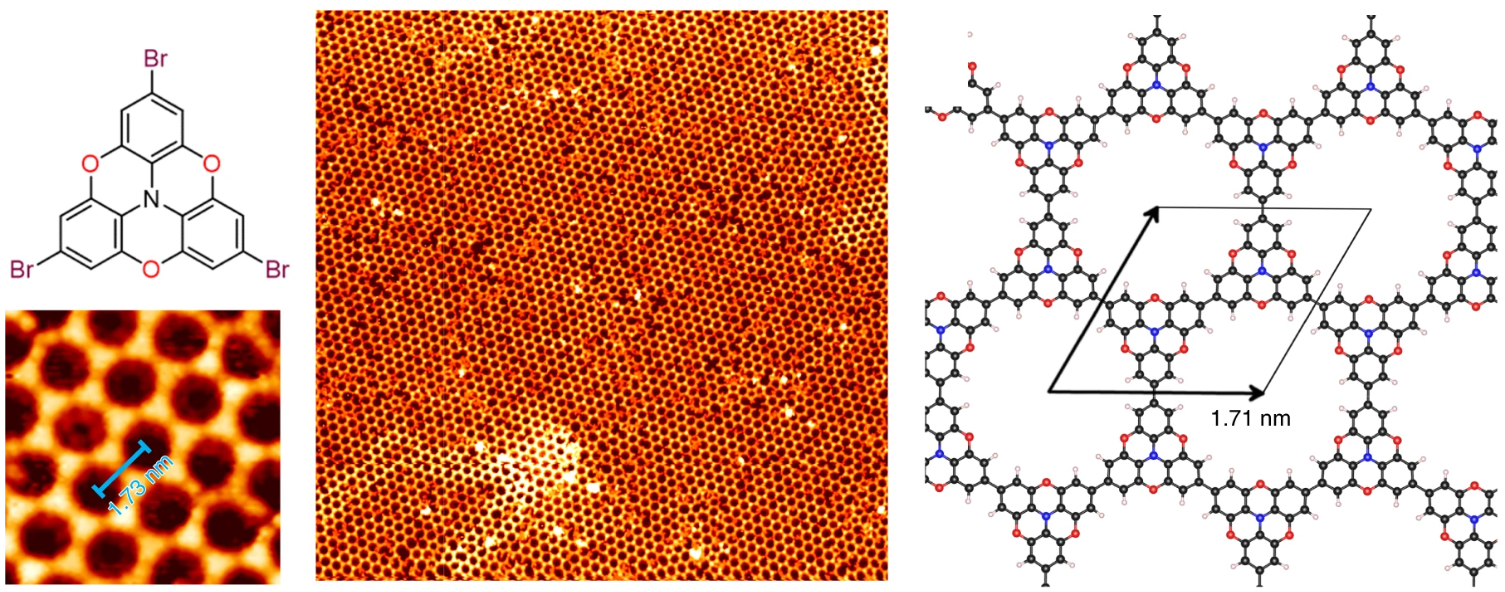
PEDOT Encapsulated and Mechanochemically Engineered Silicate Nanocrystals for High Energy Density Cathodes
M. Rasool, H.-C. Chiu, B. Zank, Y. Zeng, J. Zhou, K. Zghib, D. F. Perepichka, G. P. Demopoulos, Adv. Mater. Interfaces 2020 (DOI: 10.1002/admi.202000226)
Nitroaromatics as n-type Organic Semiconductors for Field Effect Transistors
M. R. Niazi, E. Hamzehpoor, P. Ghamari, I. F. Perepichka, D. F. Perepichka, Chem. Commun. 2020 (DOI: 10.1039/D0CC01236J)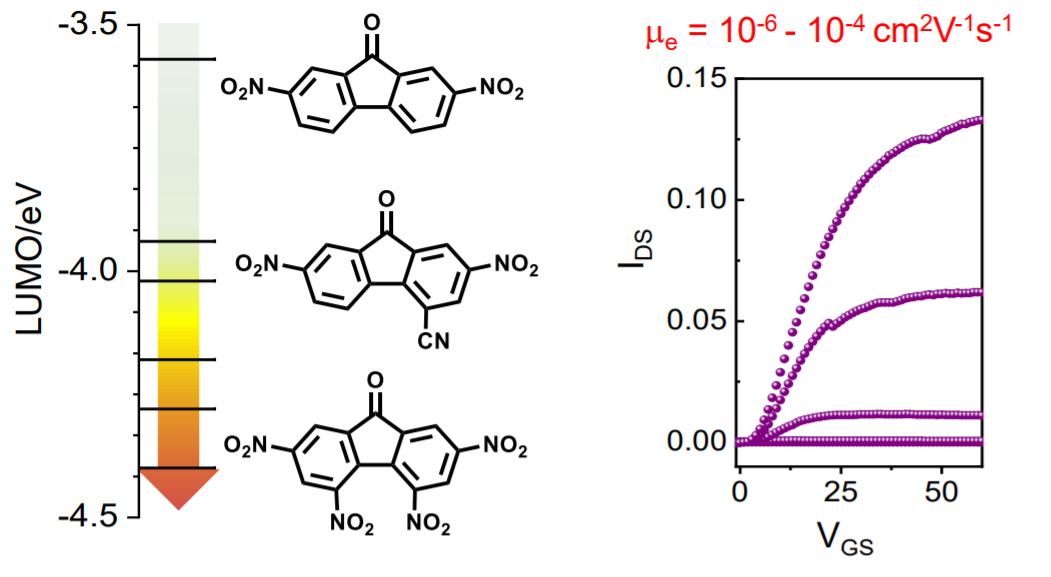
Transformation between 2D and 3D Covalent Organic Frameworks via Reversible [2+2] Cycloaddition
T. Jadhav, Y. Fang, C.-H. Liu, A. Dadvand, E. Hamzehpoor, W. Patterson, A. Jonderian, R. S. Stein, D. F. Perepichka, J. Am. Chem. Soc. 2020 (DOI: 10.1021/jacs.0c01990)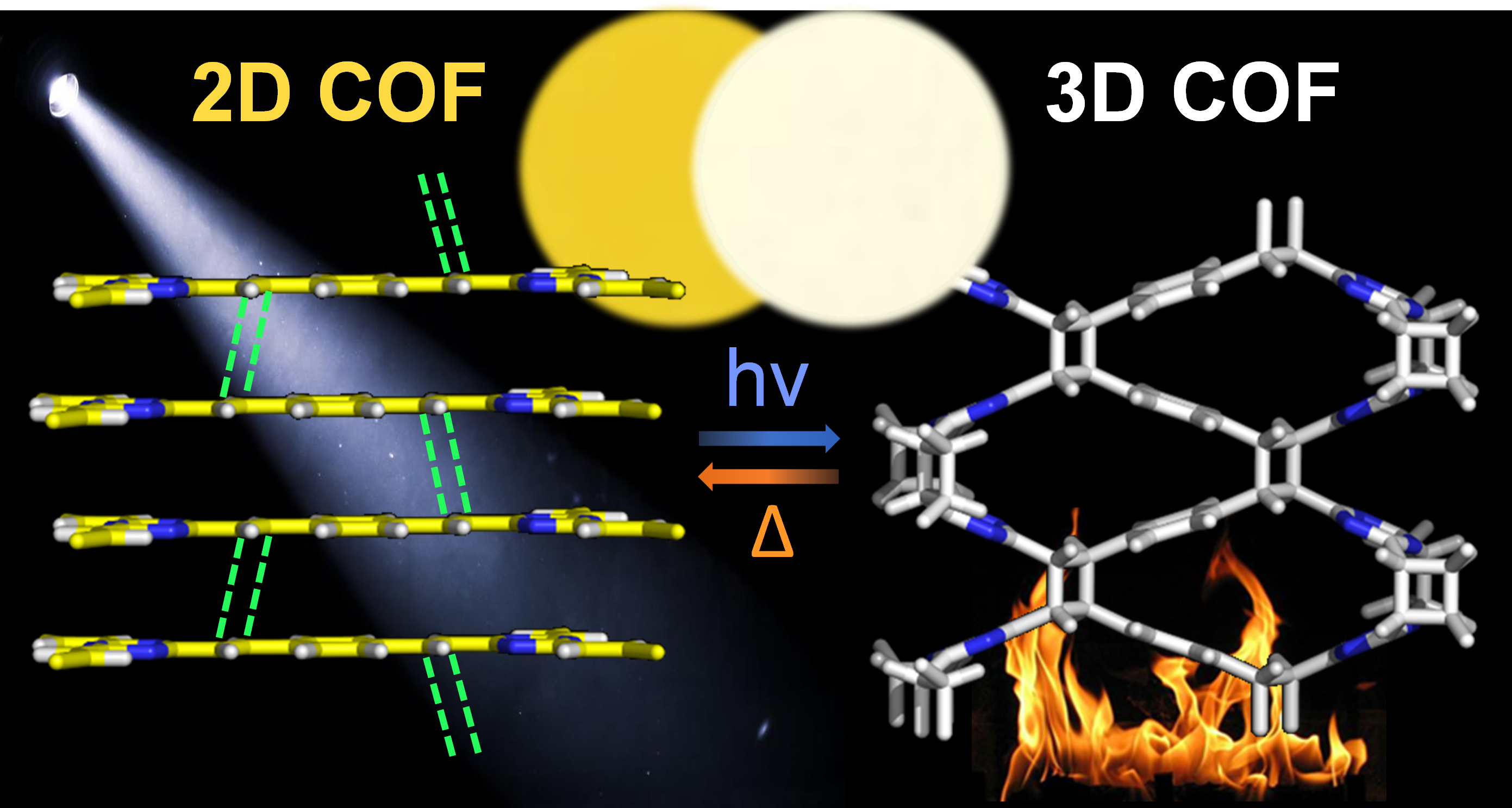
Stereospecific Epitaxial Growth of Bilayered Porous Molecular Networks
Y. Fang, B. D. Lindner, I. Destoop, T. Tsuji, Z. Zhang, R. Z. Khaliullin, D. F. Perepichka, K. Tahara, S. De Feyter, Y. Tobe, J. Am. Chem. Soc. 2020 (DOI: 10.1021/jacs.0c00108)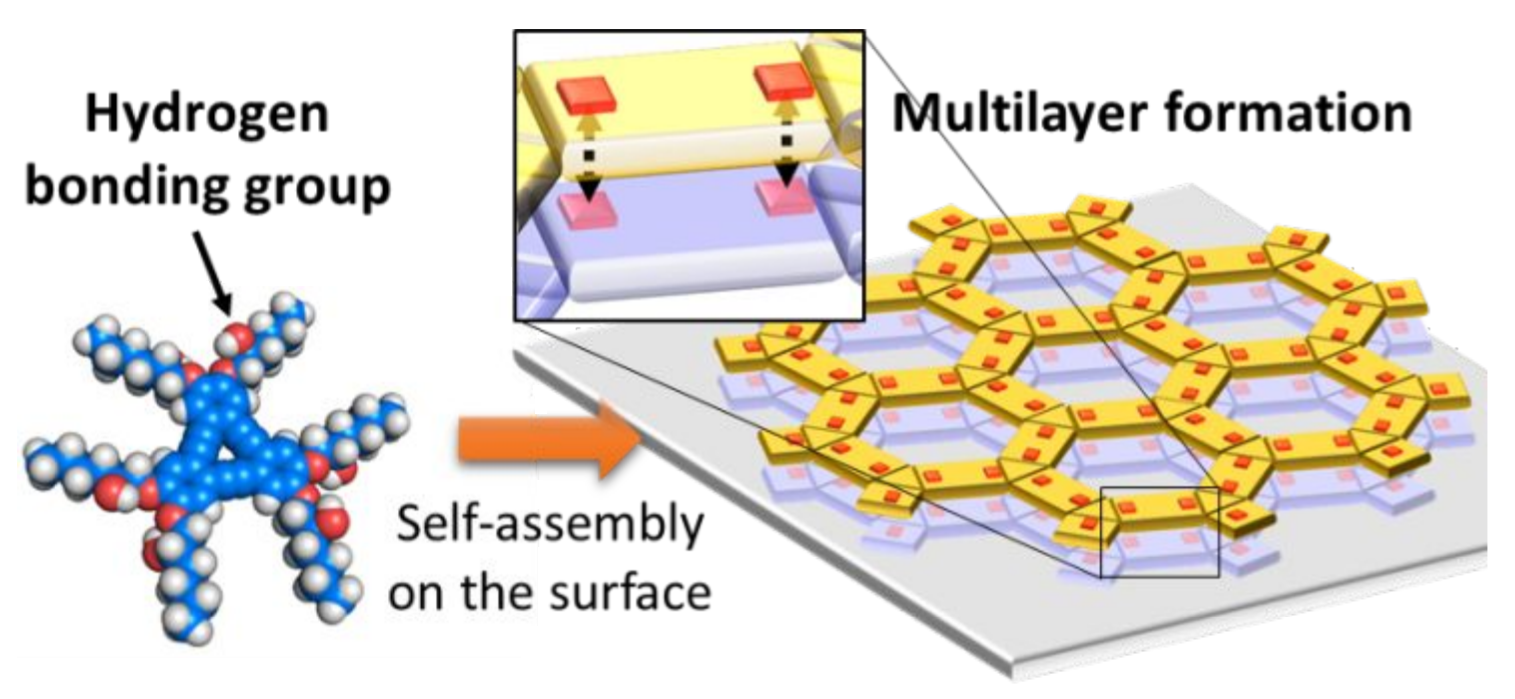
Boosting the Efficiency and Curtailing the Efficiency Roll-off in Green Perovskite Light-Emitting Diodes via Incorporating Ytterbium as Cathode Interface Layer
M. U. Ali, J. Miao, J. Cai, D. F. Perepichka, H. Yang, H. Meng, ACS Appl. Mater. Interfaces 2020 (DOI: 10.1021/acsami.0c00950)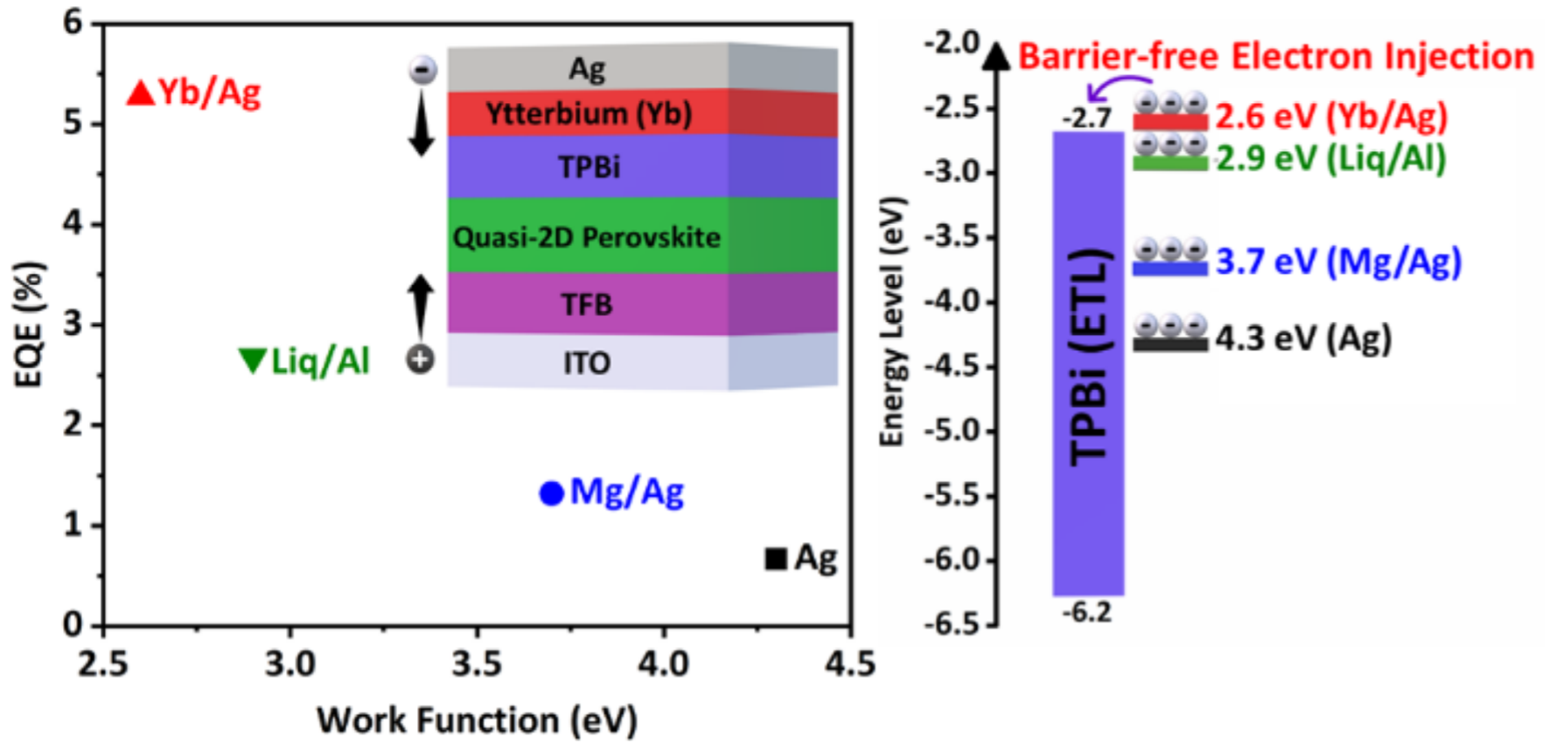
Serendipitous Formation of Semiconducting semi-NINDIGO Indigoid by Degradation of Diindolopyrrole
N. Yee, A. Dadvand, D. F. Perepichka, J. Org. Chem. 2020 (DOI: 10.1021/acs.joc.0c00054)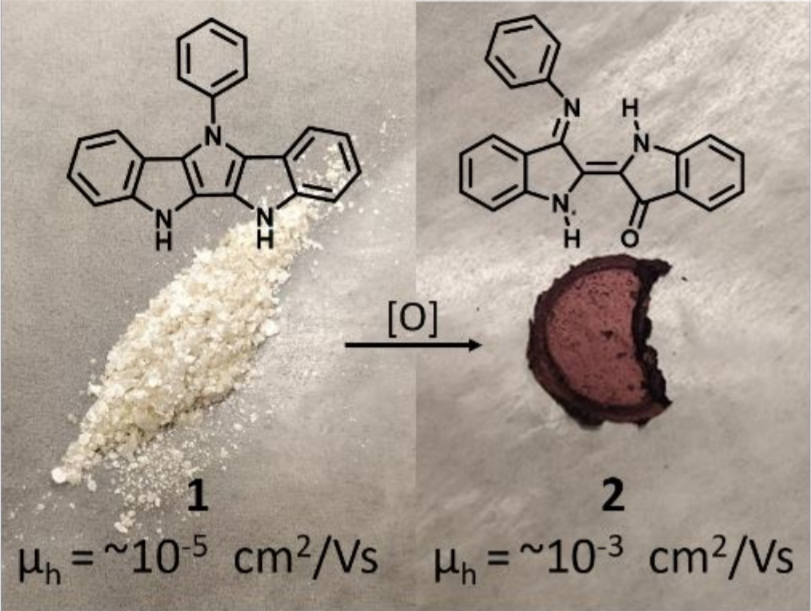
Surface-confined Single-layer Covalent Organic Frameworks: Design, Synthesis and Application
D. Cui, D. F. Perepichka, J. M., MacLeod, F. Rosei, Chem. Rev. Soc. 2020 (DOI: 10.1039/C9CS00456D)
A Trifluoromethyl Group Modified Non-fullerene Acceptor Towards Improved Power Conversion Efficiency Over 13% in Polymer Solar Cells
C. Yao, J. Zhao, Y. Zhu, B. Liu, C. Yan, D. F. Perepichka, H. Meng, ACS Appl. Mater. Interfaces 2020 (DOI: 10.1021/acsami.9b20544)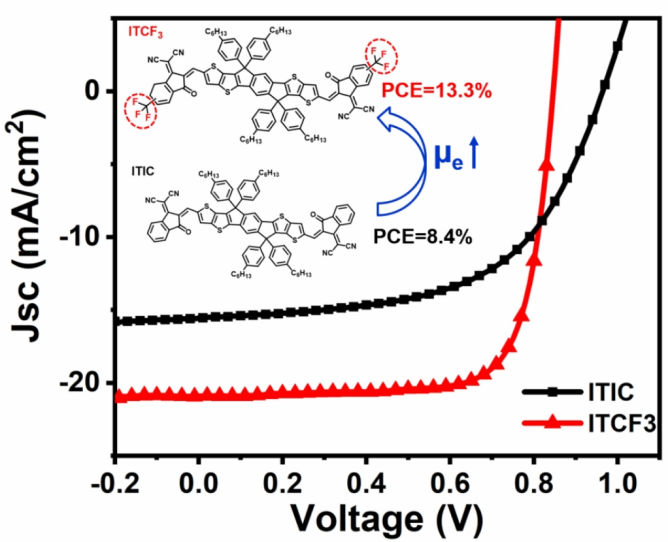
Surface-Confined Macrocyclization via Dynamic Covalent Chemistry
C. Fu, J. Mikšátko, L. Assies, V. Vrkoslav, S. Orlandi, M. Kalbác, P. Kovarícek, X. Zeng, B. Zhou, L. Muccioli, D. F. Perepichka, E. Orgiu, ACS Nano 2020 (DOI: 10.1021/acsnano.9b07671)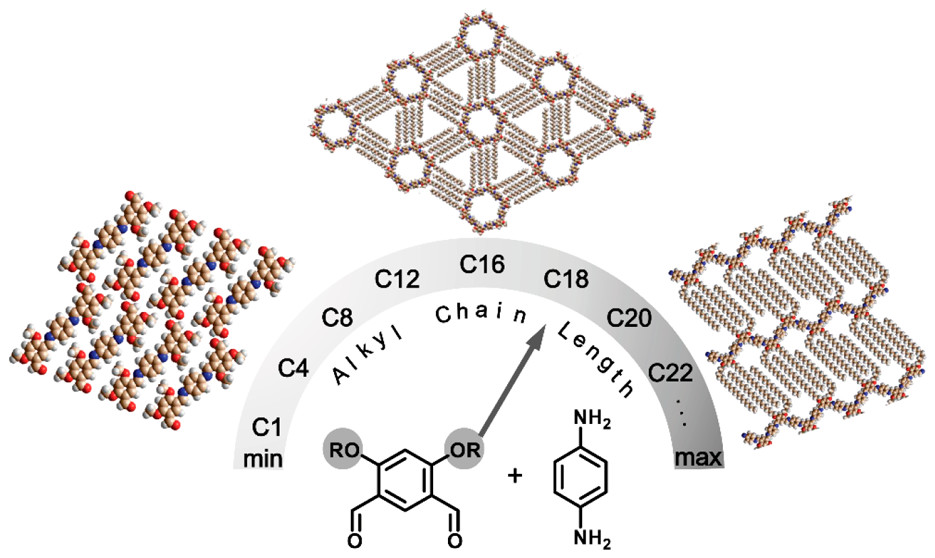
A Two-dimensional Poly(azatriangulene) Covalent Organic Framework with Semiconducting and Paramagnetic State
V. Lakshmi, C.-H. Liu, M.R. Rao, Y. Chen, F. Yuan, Dadvand A., E. Hamzehpoor, Y. Sakai-Otsuka, R.S. Stein, D.F. Perepichka, J. Am. Chem. Soc. 2020 (DOI: 10.1021/jacs.9b11528)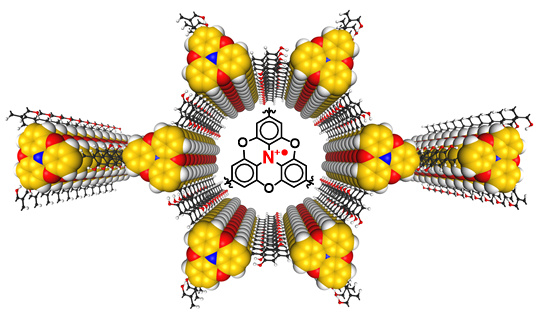
Star-shaped Triarylamine-based Hole-transport Materials in Perovskite Solar Cells
R.F. Pineda, Y. Zems, J.R. Troughton, M.R. Niazi, D.F. Perepichka, T.M. Watson, N. Robertson, Sustainable Energy Fuels 2019 (DOI: 10.1039/C9SE00366E)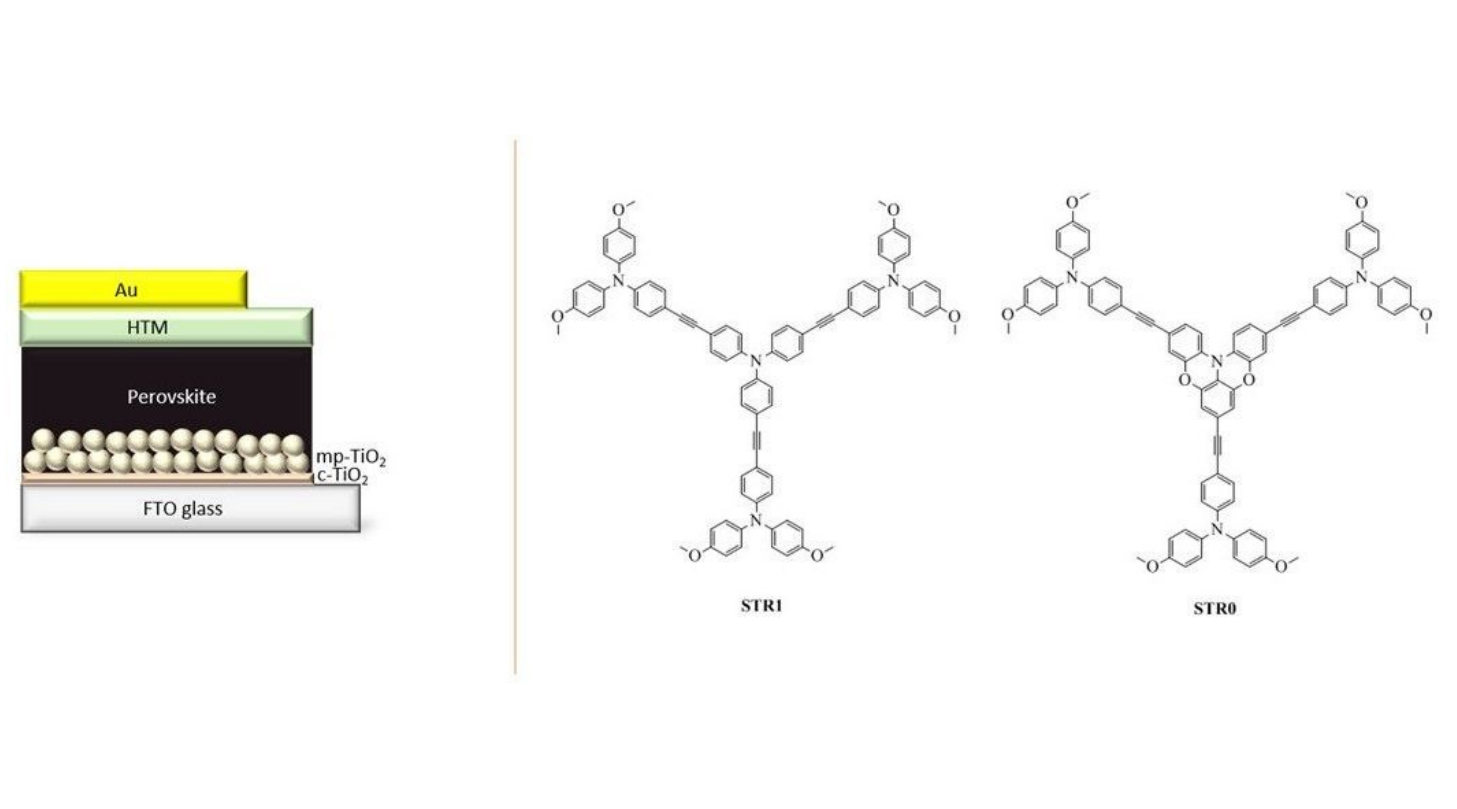
Crystal Engineering of Room Temperature Phosphorescence in Organic Solids
E. Hamzehpoor, D.F. Perepichka,
Angew. Chem. Int. Ed. 2019 (DOI: 10.1002/anie.201913393)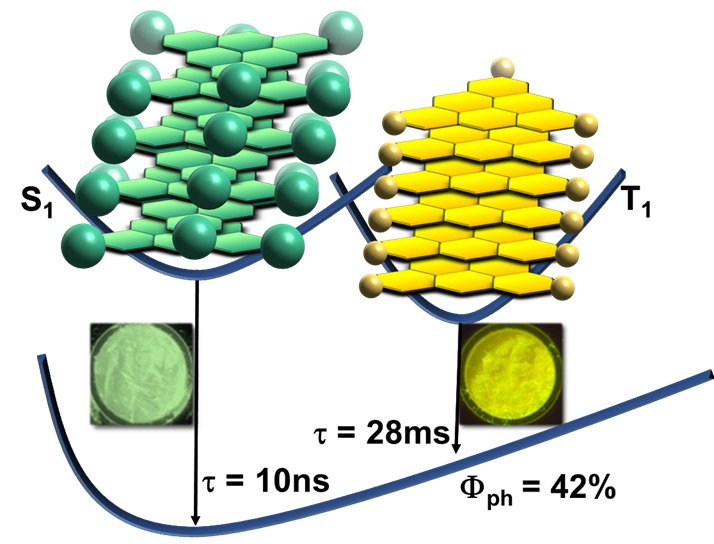
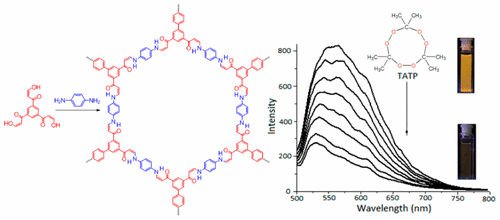
Covalent Organic Frameworks from a Monomer with Reduced Symmetry: Polymorphism and Sierpinski Triangles
D. Cui, Y. Fang, O. MacLean, D.F. Perepichka, F. Rosei., S. Clair,
Chem. Commun. 2019 (DOI: 10.1039/C9CC05674B)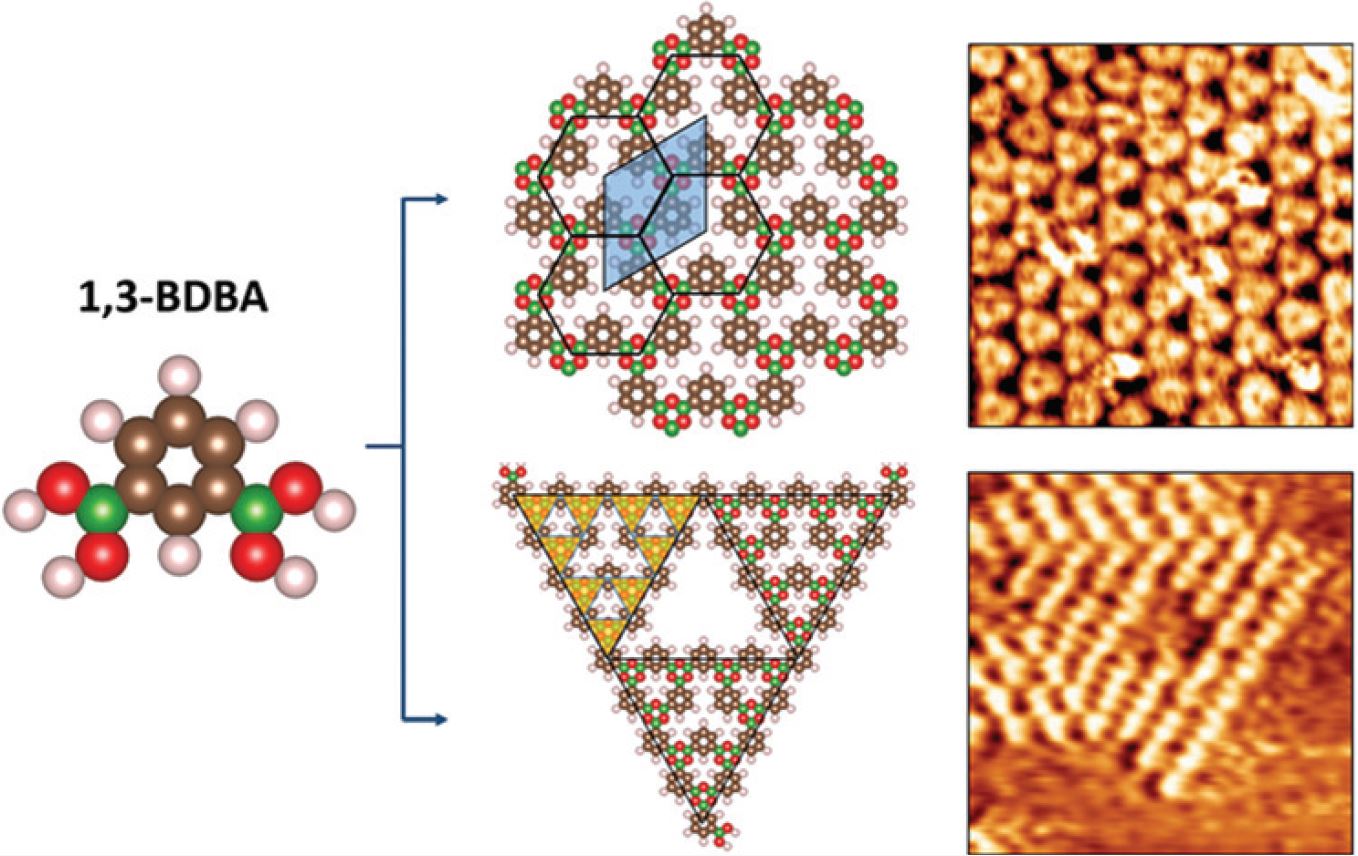
Strong Enhancement of pi-Electron Donor/Acceptor Ability by DD/AA Complementary Hydrogen Bonding
C.-H. Liu, M.R. Niazi, D.F. Perepichka,
Angew. Chem. Int. Ed. 2019, 58, 17312-17321 (DOI: 10.1002/anie.201910288)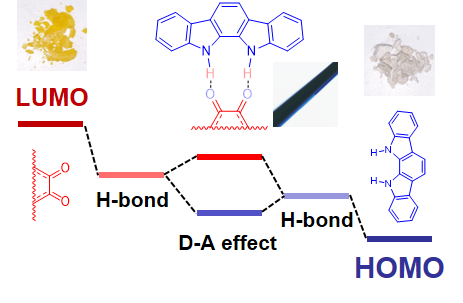
- Dynamic covalent conjugated polymer epitaxy on graphene L. Assies, C. Fu, P. Kovaricek, Z. Bastl, K.A. Drogowska, J. Lang, V.L.P. Guerra, P. Samori, E. Orgiu, D.F. Perepichka D.F., M. Kalbac, J. Mater. Chem. C 2019, 7, 12240-12247 (DOI: 10.1039/C9TC03155C)
- Temperature-induced Molecular Reorganization on Au(111) Driven by Oligomeric Defects F. De Marchi, G. Galeotti, M. Simenas, M. Gallagher, E. Hamzehpoor, O. MacLean, R. R. Malakalapalli, Y. Chen, D. Dettmann, G. Contini, E. Tornau, M. Ebrahimi, D. F. Perepichka, F. Rosei, Nanoscale 2019, 11, 19468-19476 (DOI: 10.1039/C9NR06117G)
- A Macrocyclic Oligofuran: Synthesis, Solid State Structure and Electronic Properties S.V. Mulay, O. Dishi, Y. Fang, M.R. Niazi, L.J.W. Shimon, D.F. Perepichka, O. Gidron, Chem. Sci. 2019, 10, 8527-8532 (DOI: 10.1039/C9SC03247A)
- 2D Poly(arylene vinylene) Covalent Organic Frameworks via Aldol Condensation of Trimethyltriazine T. Jadhav, Y. Fang, W. Patterson, C.-H. Liu, E. Hamzehpoor, D.F. Perepichka, Angew. Chem. Int. Ed. 2019, 58, 13753-13757 (DOI: 10.1002/anie.201906976)
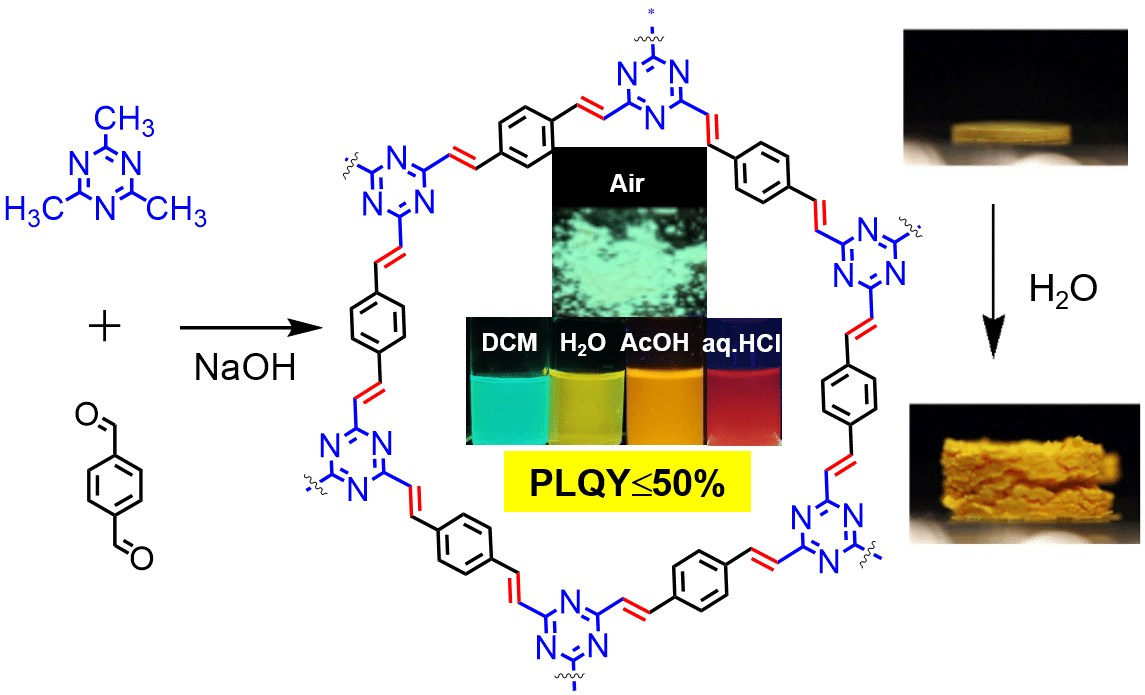
- Understanding the Photovoltaic Behavior of A–D–A Molecular Semiconductors through a Permutation of End Groups Y. Che, Y. Zhang, Y. Yang, C.-H. Liu, R. Izquierdo, S.S. Xiao, D.F. Perepichka, J. Org. Chem. 2019 (DOI: 10.1021/acs.joc.9b01654)

- Pure and Mixed Ordered Monolayers of Tetracyano-2, 6-Naphthoquinodimethane and Hexathiapentacene on the Ag (100) Surface R. Harbers, T. Heepenstrick, D.F. Perepichka, M. Sokolowski, Beilstein J. Nanotechnol. 2019, 10, 1188–1199 (DOI: 10.3762/bjnano.10.118)
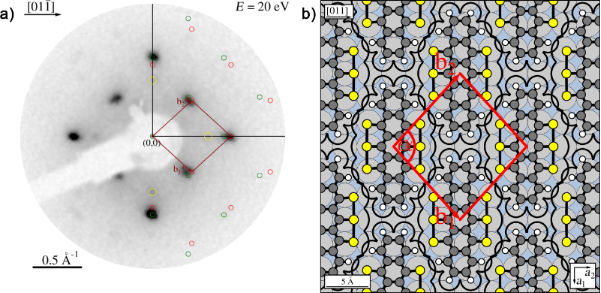
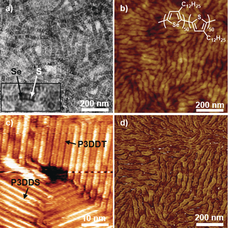
- Surface-mediated Assembly, Polymerization and Degradation of Thiophene-based Monomers G. Galeotti, F. De Marchi, T. Taerum, L.V. Besteiro, M. El Garah, J. Lipton-Duffin, M. Ebrahimi, D.F. Perepichka, F. Rosei, Chem. Sci. 2019, 10, 5167-5175 (DOI: 10.1039/C8SC05267K)
- Polysiloxane–poly (vinyl alcohol) Composite Dielectrics for High-efficiency Low Voltage Organic Thin Film Transistors J. Cao, X. Wei, Y. Che, A. Li, Y. He, C. He, Y. Zhu, X. Chen, T. Li, I. Murtaza, L. Yan, D.F. Perepichka, H. Meng, J. Mater. Chem. C 2019 7, 4879-4886 (DOI: 10.1039/C9TC00717B)
- An Unexpected Organometallic Intermediate in Surface-confined Ullmann Coupling G. Galeotti, M. Di Giovannantonio, A. Cupo, S. Xing, J. Lipton-Duffin, M. Ebrahimi, G. Vasseur, B. Kierren, Y. Fagot-Revurat, D. Tristant, V. Meunier, D.F. Perepichka, F. Rosei, G. Contini Nanoscale 2019 11, 7682-7689 (DOI: 10.1039/C9NR00672A)
- Supramolecular Assemblies on Surfaces: Nanopatterning, Functionality, and Reactivity D.P. Goronzy, M. Ebrahimi, F. Rosei, Arramel, Y. Fang, S. De Feyter, S. L. Tait, C. Wang, P. H Beton, A.T.S. Wee, P.S. Weiss, D.F. Perepichka, ACS Nano 2018 12, 7445-7481 (DOI: 10.1021/acsnano.8b03513)

- Alkyl Chain Length Effects on Double-deck Assembly at a Liquid/Solid Interface Y. Fang, M. Cibian, G. S. Hanan, D. F. Perepichka, S. D. Feyter, L. A. Cuccia, O. Ivansenko, Nanoscale 2018 10, 14993-15002 (DOI: 10.1039/C8NR04220A)
- Face-on vs. Edge-on: Tuning the Structure of Tetrathiafulvalene Monolayers with Solvent C. Fu, E. Orgiu, D. F. Perepichka, J. Mater. Chem. C 2018 6, 3787-3791. (DOI: 10.1039/C7TC05757A)
- A 2D Substitutional Solid Solution Through Hydrogen-Bonding of Molecular Building Blocks J. M. MacLeod, J. Lipton-Duffin, C. Fu, T. Taerum, D. F. Perepichka, F. Rosei, ACS Nano 2017 11 (9), 8901–8909 DOI: 10.1021/acsnano.7b03172.
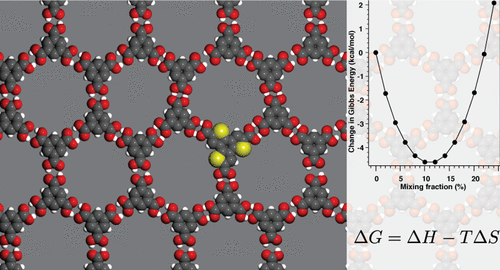
- The Role of Halogens in On-Surface Ullmann Polymerization, G. Galeotti, M. Di Giovannantonio, J. Lipton-Duffin, M. Ebrahimi, S. Tebi, A. Verdini, L. Floreano, Y. Fagot-Revurat, D. F. Perepichka, F. Rosei, G. Contini, Faraday Trans. 2017, DOI: 10.1039/C7FD00099E
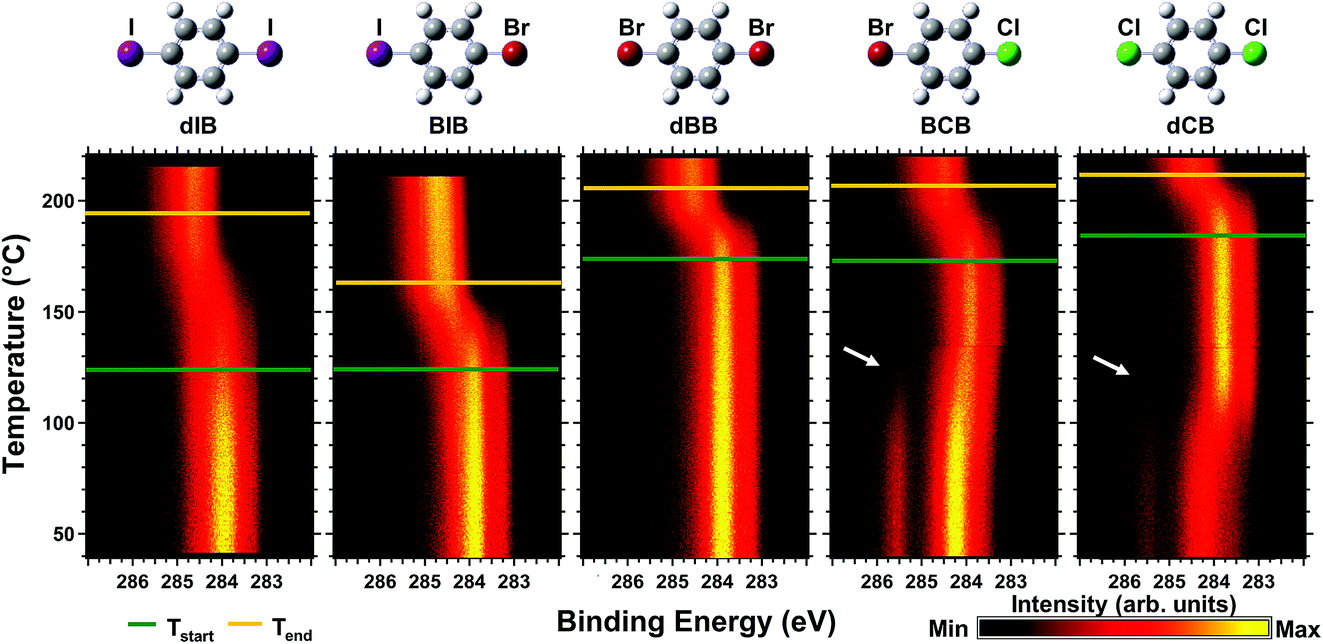
- A Wide Bandgap Naphthalene Semiconductor for Thin-Film Transistors, L. Yan, F. Popescu, M. R. Rao, H. Meng, D. F. Perepichka, Adv. Electron. Mater. 2017, 3, 1600556(1-8).
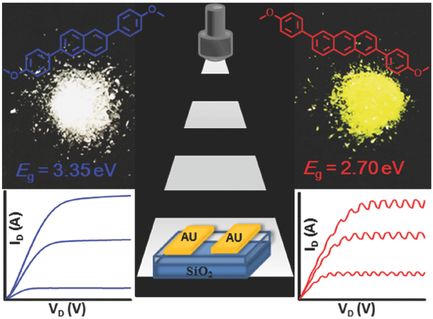
- H-bonding Control of Supramolecular Ordering of Diketopyrrolopyrroles, C. Fu, P. J. Beldon, D. F. Perepichka, Chem. Mater. 2017, 29, 2979–2987.
- Patchy Nanofibers from the Thin Film Self-Assembly of a Conjugated Diblock Copolymer, E. Kynaston, Y. Fang, J. G. Manion, N. K. Obhi, J. Y. Howe, D. F. Perepichka, D. S. Seferos, Angew. Chem. Int. Ed. 2017, 56, 6152–6156.
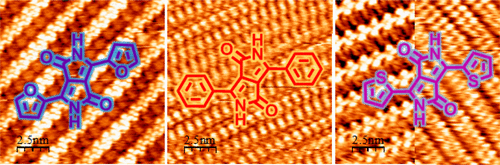
- Conjugated Covalent Organic Frameworks via Michael Addition-Elimination, M. R. Rao, Y. Fang, S. De Feyter, D. F. Perepichka, J. Am. Chem. Soc., 2017, 139, 2421–2427. (DOI: 10.1021/jacs.6b12005)
- Mechanistic Picture and Kinetic Analysis of Surface-Confined Ullmann Polymerization, M. Di Giovannantonio, M. Tomellini, J. Lipton-Duffin, G. Galeotti, M. Ebrahimi, A. Cossaro, A. Verdini, N. Kharche, V. Meunier, G. Vasseur, Y. Fagot-Revurat, D. F. Perepichka, F. Rosei, G. Contini, J. Am. Chem. Soc., 2016, 138, 16696–16702. (DOI: 10.1021/jacs.6b09728)
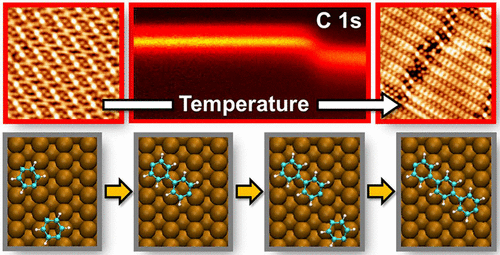
- A Smart Polymer with a High Sensitivity to Temperature and Humidity Based on Polyacrylamide Hydrogel Doped with Polyiodide, H. Yu, Y. Guo, C. Yao, D. F. Perepichka, H. Meng, J. Mater. Chem. C, 2016, 4, 11055–11058. (DOI: 10.1039/C6TC04200G)
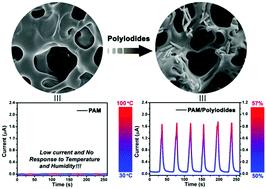
- Controlling C60 Organization through Dipole-Induced Band Alignment at Self-Assembled Monolayer Interfaces, M. A. Mezour, O. Voznyy, E. Sargent, R. B. Lennox, D. F. Perepichka, Chem. Mater., 2016, 28, 8322–8329. (DOI: 10.1021/acs.chemmater.6b03527)
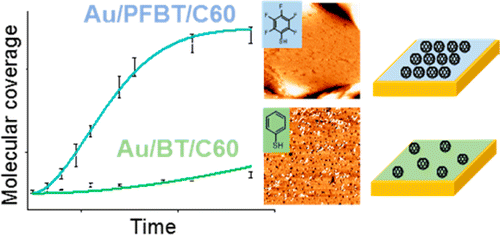
- Synthesis of Macrocyclic Poly(3-hexylthiophene) and Poly(3-heptylselenophene) by Alkyne Homocoupling, G. R. McKeown, Y. Fang, N. K. Obhi, J. G. Manion, D. F. Perepichka, D. S. Seferos, ACS Macro Lett. , 2016, 5, 1075. (editor’s choice and cover page) (DOI:10.1021/acsmacrolett.6b00603)
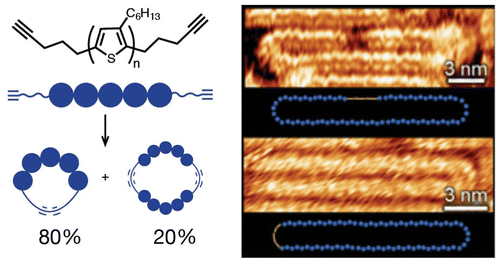
- Flexible Asymmetric Supercapacitors via Spray-Coating of a New Electrochromic Donor-Acceptor Polymer, Y. Guo, W. Li, H. Yu, D. F. Perepichka, H. Meng, Adv. Ener. Mater. 2016, 1601623. (DOI:10.1002/aenm.201601623)
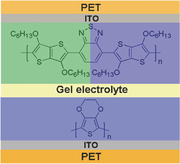
- Hydrogen Bonding vs Molecule-Surface Interactions in 2D Self-Assembly of [C60]fullerenecarboxylic acids, M. A. Mezour, R. M. Choueiri, O. Lukoyanova, R. B. Lennox, D. F. Perepichka, Nanoscale 2016, 8, 16955 (DOI:10.1039/C6NR04115A)
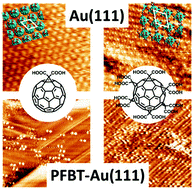
- Complementary Hydrogen Bonding Modulates Electronic Properties and Controls Self-Assembly of Donor/Acceptor Semiconductors, H. T. Black, N. Yee, Y. Zems, D. F. Perepichka, Chem. Eur. J. 2016,22, 17251.(DOI:10.1002/chem.201602543)
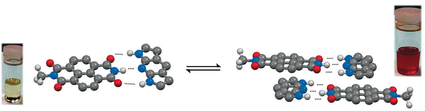
- A Molecular Necklace: Threading b-Cyclodextrins onto Polymers Derived from Bile Acids, Y.-G. Jia, C. Malveau, M. A. Mezour, D. F. Perepichka, X. X. Zhu, Angew. Chem. Int. Ed., 2016, 128, 12158-12162. (DOI: 10.1002/ange.201605090)
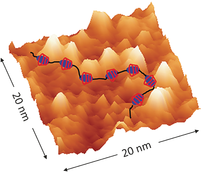
- Aromatization of Benzannulated Perylene-3,9-diones: Unexpected Photophysical Properties and Reactivity, M. Rajeswara Rao, Shea Johnson, Dmitrii F. Perepichka, Org. Lett. 2016, 18, 3574. (DOI:10.1021/acs.orglett.6b01559)
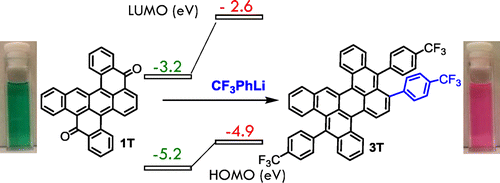
- A new approach to polycyclic azaarenes: visible-light photolysis of vinyl azides in the synthesis of diazabenzopyrene and diazaperylene, J. A. Schneider, D. F. Perepichka, J. Mater. Chem. C 2016, 4, 7269. (DOI: 10.1039/C6TC02046A)
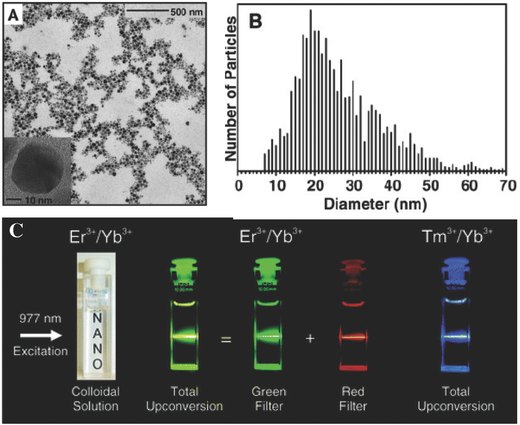
- Lanthanide Ion Doped Upconverting Nanoparticles: Synthesis, Structure and Properties, C. Yan, H. Zhao, D. F. Perepichka, F. Rosei, Small 2016, 12, 3888-3907. (DOI:10.1002/smll.201601565)
- Influence of Heteroatoms on the Charge Mobility of Anthracene Derivatives, L. Yan, Y. Zhao, H. Yu, Z. Hu, Y. He, O. Goto, C. Yan, T. Chen, R. Chen, Y.-L. Loo, D. F. Perepichka, H. Meng. W. Huang, J. Mater. Chem. C 2016, 4, 3517. (DOI: 10.1039/C6TC01088A)
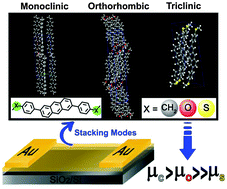
- Supramolecular ordering of difuryldiketopyrrolopyrrole: the effect of alkyl chains and inter-ring twisting, C. Fu, F. Belanger-Gariepy, D. F. Perepichka, Cryst. Eng. Comm. 2016, 18, 4285. (DOI: 10.1039/C6CE00383D)
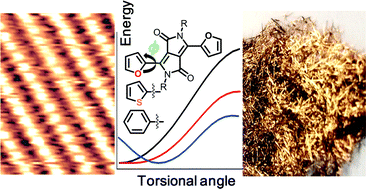
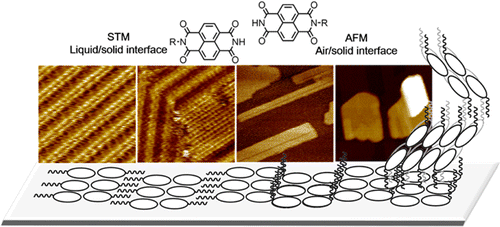
- Unravelling the self-Assembly of hydrogen bonded semiconductors in 2D and 3D, C. Fu, H.-P. Lin, J. L. Macleod, A. Krayev, F. Rosei, D. F. Perepichka, Chem. Mater. 2016, 28, 951. (DOI: 10.1021/acs.chemmater.5b04706)
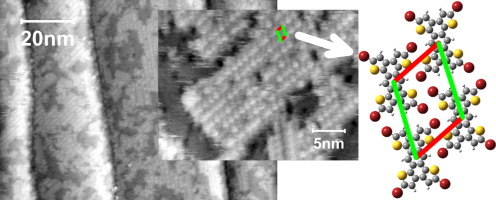
- Supramolecular structures of halogenated oligothiophenes on Si(111)-√3x√3-Ag surface, R. Liu, C. Fu, D. F. Perepichka, M. C. Gallagher, Surf. Science 2016, 647, 51. (DOI: 10.1016/j.susc.2015.12.001)
- Quasi one-Dimensional Band Dispersion and Surface Metallization in Long Range Ordered Polymeric Wires, G. Vasseur, Y. Fagot-Revurat, M. Sicot, B. Kierren, D. Malterre, L. Cardenas, G. Galeotti, J. Lipton-Duffin, F. Rosei, M. Di Giovannantonio, G. Contini, P. Lefevre, F. Bertran, V. Meunier, L. Liang, D. F. Perepichka, Nat. Comm. 2016, 7, 10235. (DOI: 10.1038/ncomms10235)
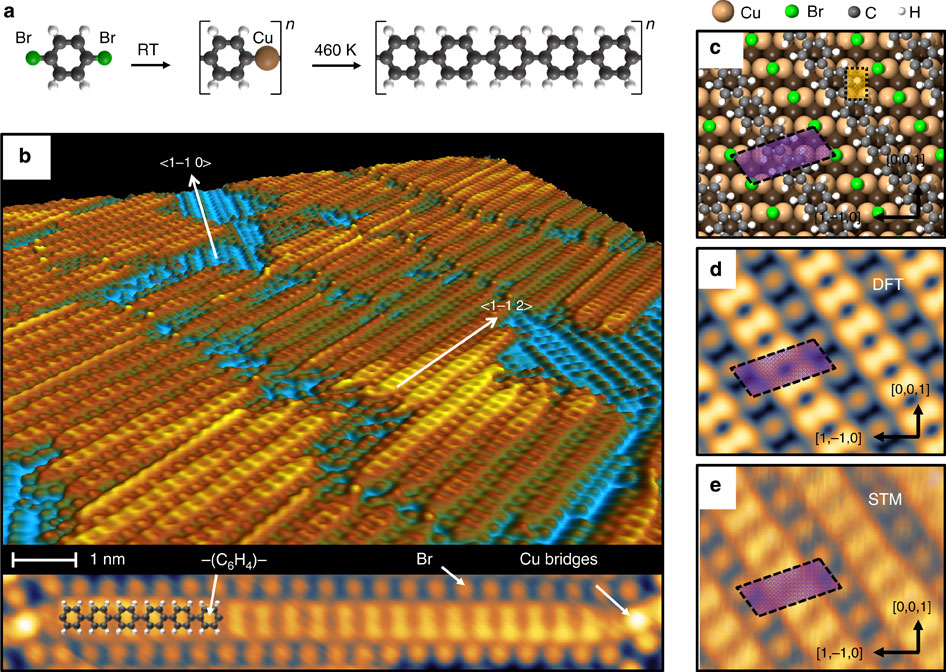
- Solution and air stable host/guest architectures from a single layer covalent organic framework, D. Cui, J. L. Macleod, M. Ebrahimi, D. F. Perepichka, F. Rosei, Chem. Commun. 2015, 51, 16510–16513. (DOI: 10.1039/C6TC04200G)
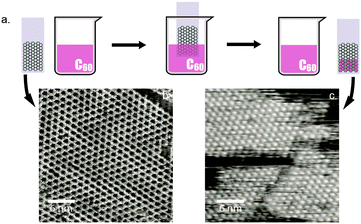
- Tailoring the Reaction Path in the On-Surface Chemistry in Thienoacenes, L. E. Dinca, J. L. Macleod, J. Lipton-Duffin, C. Fu, D. Ma, D. F. Perepichka, F. Rosei, J. Phys. Chem. C 2015, 119, 22432-22438. (DOI: 10.1021/acs.jpcc.5b05418)
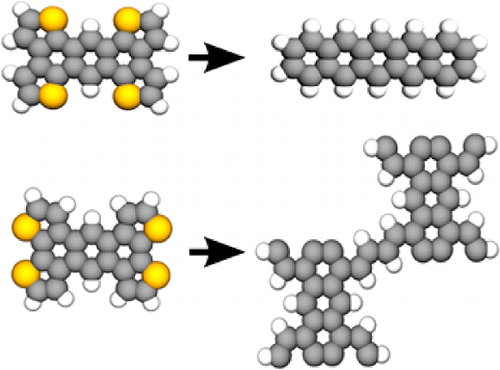
- Synthesis and Divergent Electronic Properties of Two Ring-Fused Derivatives of 9,10-Diphenylanthracene, M. R. Rao, H. T. Black, D. F. Perepichka, Org. Lett. 2015, 17, 4224–4227 (DOI: 10.1021/acs.orglett.5b02009)
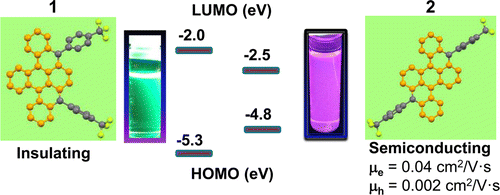

- Polymorphism in New Thienothiophene-Thiazolothiazole Organic Semiconductor, M. R. Rao, Y. Fang, S. De Feyter, D. F. Perepichka, ChemPhysChem, 2015, 6, 1173-1178 (Invited paper for Special Issue; cover page) (DOI: 10.1002/cphc.201590028)
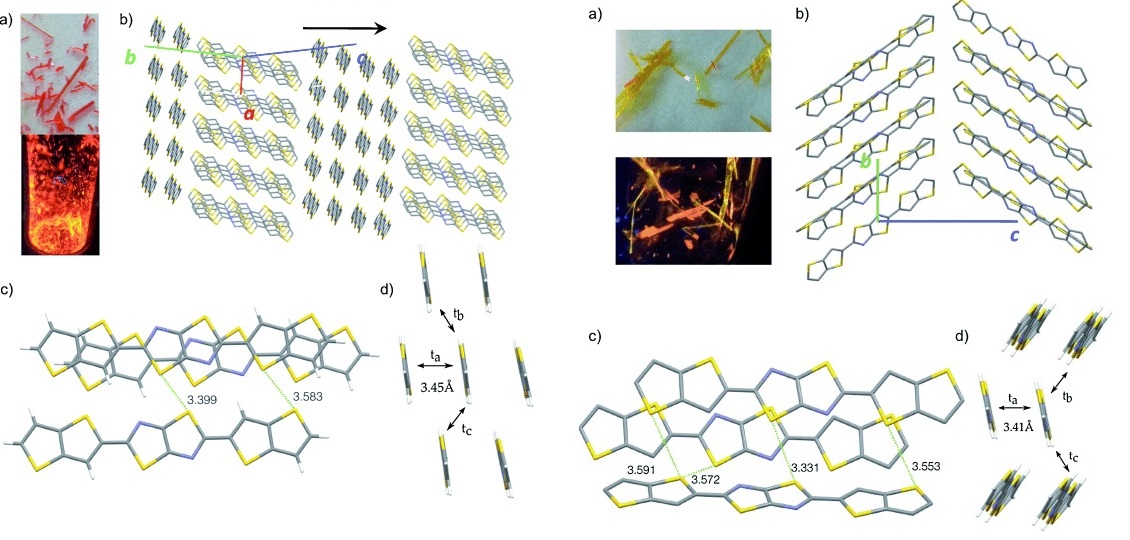
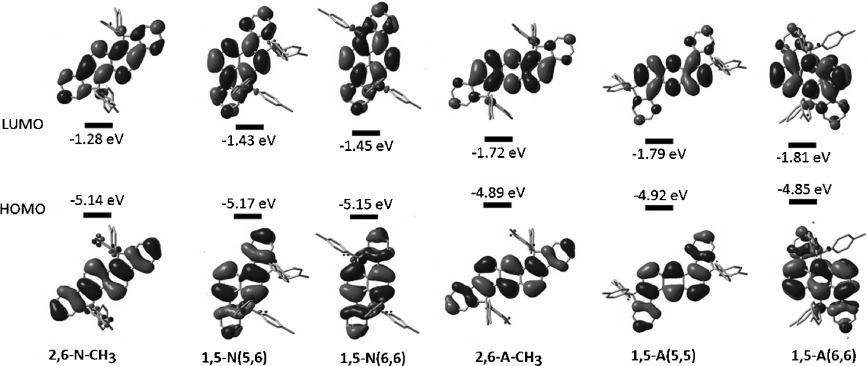
- pi--Extended Indenofluorenes, M. R. Rao, A. Desmecht, D. F. Perepichka, Chem. Eur. J., 2015, 21, 6193-6201. (DOI: 10.1002/chem.201406646)
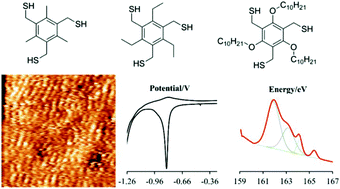
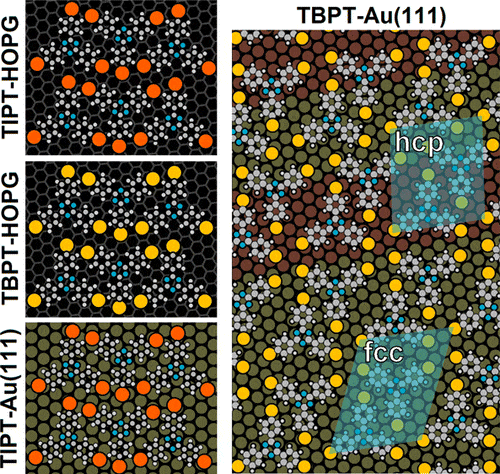
- Tridentate Benzylthiols on Gold(111): Control of Self-Assembly Geometry, M. A. Mezour, I. I. Perepichka, O. Ivasenko, R. B. Lennox, D. F. Perepichka, Nanoscale, 2015, 7, 5014-5022. (DOI: 10.1039/C4NR07207C)
- Substrate, molecular structure and solvent effects in 2D self-assembly via hydrogen and halogen bonding, R. Gatti, J. M. Macleod, J. A. Lipton-Duffin, A. Moiseev, D. F. Perepichka, J. Phys. Chem. C, 2014, 118, 25505-25516 (DOI: 10.1021/jp507729w)
- High thermal stability of block-copolymer capped Au and Cu nanoparticles, I. I. Perepichka, M. A. Mezour, D. F. Perepichka, R. B. Lennox, Chem. Comm., 2014, 50, 11919–11921 (DOI: 10.1039/C4CC04937C)
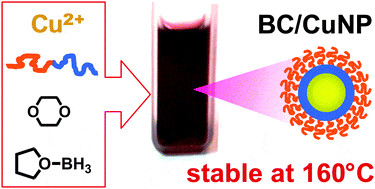
- Supramolecuar control of organic p/nheterojunctions by complementary hydrogen bonding, H.T. Black, H. Lin, F. Bélanger-Gariépy, D. F. Perepichka, Faraday Discuss., 2014, 174, 297-312. (DOI: 10.1039/C4FD00133H)
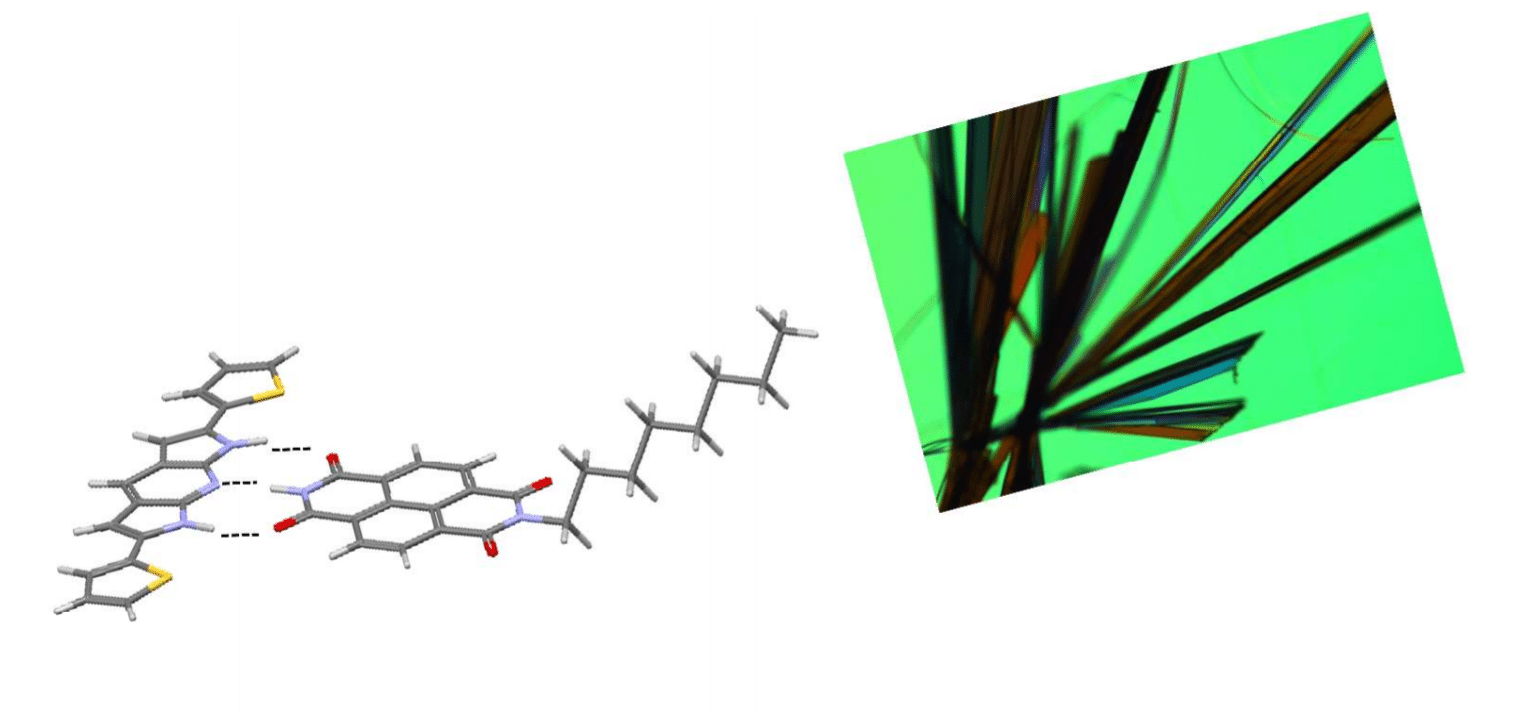
- Tip-induced C-H activation and oligomerization of thienoanthracenes , L. E. Dinca, J. M. MacLeod, J. Lipton-Duffin, C. Fu, D. Ma, D. F. Perepichka, F. Rosei, Chem. Comm., 2014, 50, 8791–8793. (DOI: 10.1039/C4CC03719G)
- Advances and Challenges in Synthesis of Poly(p-phenylene vinylene)-Based Polymers, A. Blayney, I.F. Perepichka, F. Wudl, D.F. Perepichka Isr. J. Chem., 2014, 54, 674-688. (DOI: 10.1002/ijch.201400067 )
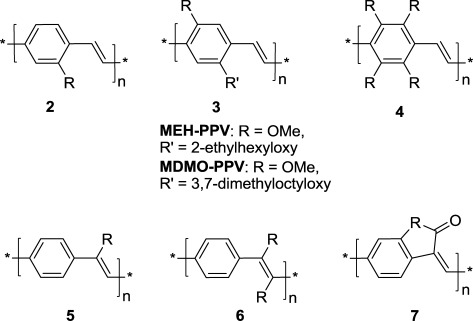
- Pentacenodithiadiazoledione, an n-type semiconductors for field effect transistors, Z. Shi, H.T. Black, A. Dadvand, D.F. Perepichka, J. Org. Chem., 2014, 79, 5858–5860. (DOI: 10.1021/jo500760c)
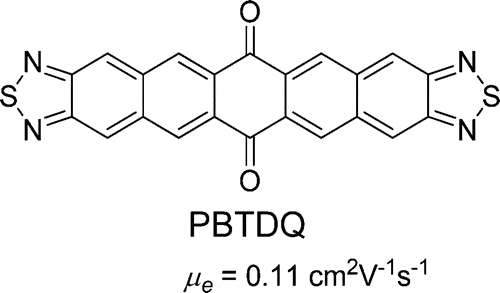
- Dithienonaphthothiadiazole Semiconductors: Synthesis, Properties, and Application to Ambipolar Field Effect Transistors, Q. Shuai, H. T. Black, A. Dadvand, D. F. Perepichka, J. Mater. Chem. C, 2014, 2, 3972–3979. (DOI: 10.1039/C4TC00094C)
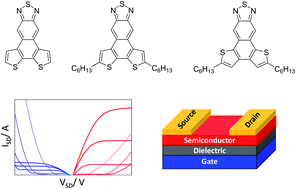
- Directing the Assembly of Gold Nanoparticles with Two-Dimensional Molecular Networks, M. A. Mezour, I. I. Perepichka, J. Zhu, R. B. Lennox, D. F. Perepichka, ACS Nano, 2014, 8, 2214–2222. (DOI: 10.1021/nn405357j)
- Reply to the comment by Fei Song to our paper “Insight into organometallic intermediate and its evolution to covalent bonding in surface-confined Ullmann polymerization", M. Di Giovannantonio, M. El-Garah, J. Lipton-Duffin, V. Meunier, L. Cardenas, Y. Fagot-Revurat, A. Cossaro, A. Verdini, D. F. Perepichka, F. Rosei, G. Contini, ACS Nano, 2014, 8, 1969–1971.(DOI: 10.1021/nn500322r)

- Crystal Engineering of Dual Channel p/n Organic Semiconductors by Complementary Hydrogen Bonding, H. T. Black, D. F. Perepichka, Angew. Chem. Int. Ed., 2014, 53, 2138–2141 (DOI: 10.1002/anie.201310902)
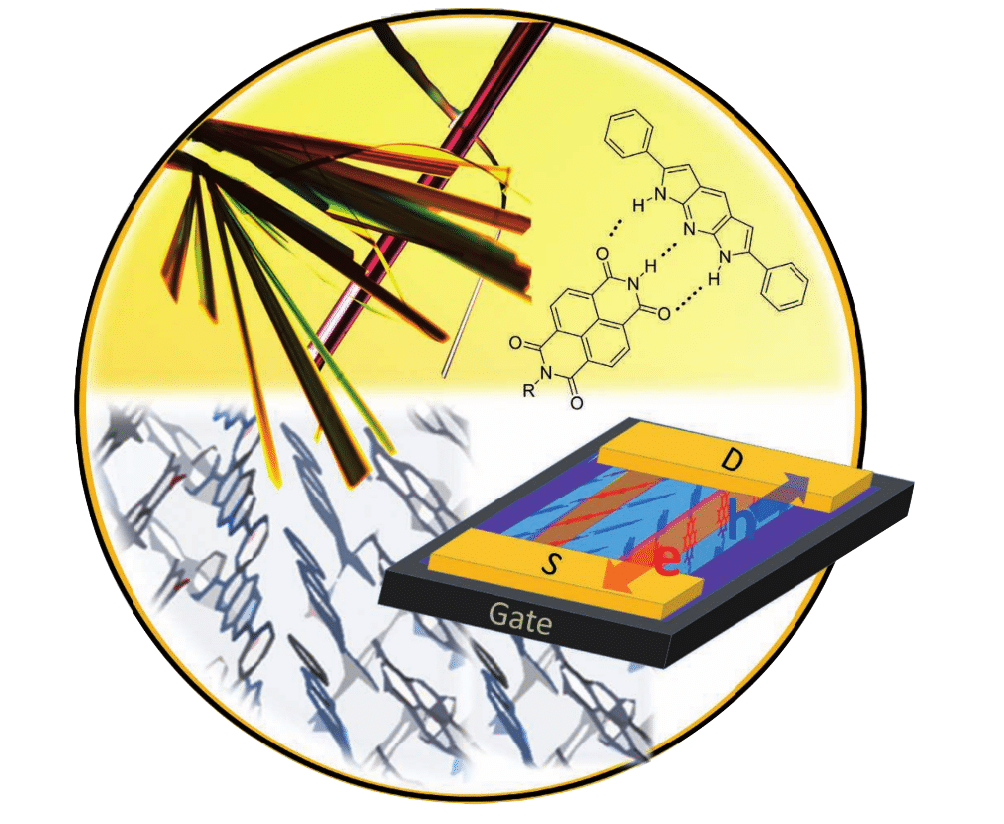
- Ullmann-Type Coupling of Brominated Tetrathienoanthracene on Crystalline Copper and Silver,, R. Gutzler, L. Cardenas, J. Lipton-Duffin, M. El Garah, L. E. Dinca, C. E. Szakacs, C. Fu, M. Gallagher, M. Vondracek, M. Rybachuk, D. F. Perepichka, F. Rosei Nanoscale, 2014, 6, 2660–2668. (DOI: 10.1039/C3NR05710K)
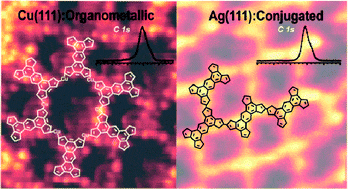
- Protecting the triplet state in sterically congested platinum porphyrin, A. G. Moiseev, E. A. Margulies, J. A. Schneider, F. Belanger-Gariepy, D. F. Perepichka, Dalton Trans., 2014, 43, 2676–2683. (DOI: 10.1039/C3DT52926F)
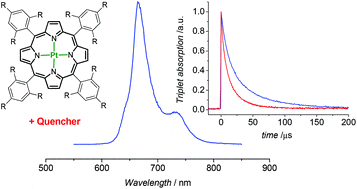
- Tuning the Electronic Properties of Poly(thienothiophene vinylene)s via Alkylsulfanyl and Alkylsulfonyl Substituents, J. A. Schneider, A. Dadvand, W. Wen, D. F. Perepichka, Macromolecules, 2013, 46, 9231–9239 (DOI: 10.1021/ma402018n)
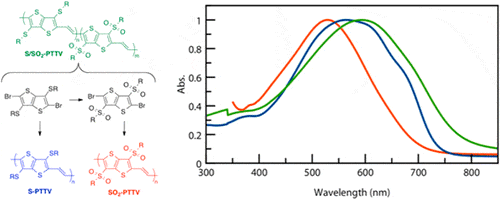
- Convenient synthesis of a highly soluble and stable phosphorescent platinum porphyrin dye, Y. Zems, A.G. Moiseev, D.F. Perepichka, Org. Lett., 2013, 15, 5330-5333 (DOI: 10.1021/ol402590c)

- pi-Electron conjugation in two dimensions, R. Gutzler, D. F. Perepichka, J. Am. Chem. Soc., 2013, 135, 16585-16594 (DOI: 10.1021/ja408355p)

- Insight into organometallic intermediate and its evolution to covalent bonding in surface-confined Ullmann polymerization, M. Di Giovannantonio, M. El Garah, J. Lipton-Duffin, V. Meunier, L. Cardenas, Y.F. Revurat, A. Cossaro, A. Verdini, D.F. Perepichka, F. Rosei, G. Contini, ACS Nano, 2013, 7, 8190-8198 (DOI: 10.1021/nn4035684)

- Two-dimensional self-assembly of a symmetry-reduced tricarboxylic acid, J.M. MacLeod, Z.B. Chaouch, D.F. Perepichka, F. Rosei, Langmuir, 2013, 29, 7318-7324 (DOI: 10.1021/la3047593)

- Unprecedented transformation of tetrathienoanthracene into pentacene on Ni (111), L.E. Dinca, C. Fu, J.M. MacLeod, J. Lipton-Duffin, J.L. Brusso, C.E. Szakacs, D. Ma, D.F. Perepichka, F. Rosei, ACS Nano, 2013, 7, 1652-1657 (DOI: 10.1021/nn305572s)

- Perfluoroalkyl-substitution versus electron-deficient building blocks in design of oligothiophene semiconductors, H.T. Black, A. Dadvand, S. Liu, V.S. Ashby, D.F. Perepichka, F. Rosei, J. Mater. Chem. C, 2013, 1, 260-267 (DOI: 10.1039/C2TC00032F)
- 1, 5-, 2, 6-and 9, 10-distyrylanthracenes as luminescent organic semiconductors, A. Dadvand, W.H. Sun, A.G. Moiseev, F. Bélanger-Gariépy, F. Rosei, H. Meng, D. F. Perepichka, J. Mater. Chem. C., 2013, 1, 2817-2825 (DOI: 10.1039/C3TC30247D)
- Oligofuran-containing molecules for organic electronics, O. Gidron, A. Dadvand, E.W.H. Sun, I. Chung, L.J.W. Shimon, M. Bendikov, D. F. Perepichka, J. Mater. Chem. C., 2013, 1, 4358-4367 (DOI: 10.1039/C3TC00079F)
- Synthesis and electronic structure of a two dimensional pi-conjugated polythiophene, L. Cardenas, R. Gutzler, J. Lipton-Duffin, C. Fu, J.L. Brusso, L.E. Dinca, M. Vondráček, Y. Fagot-Revurat, D. Malterre, F. Rosei, D.F. Perepichka, Chem. Sci., 2013, 4, 3263-3268 (DOI: 10.1039/C3SC50800E)
- Halogen bonds in 2D supramolecular self-assembly of organic semiconductors, R. Gutzler, C. Fu, A. Dadvand, Y. Hua, J. MacLeod, F. Rosei*, D. F. Perepichka*, Nanoscale, 2012, 4, 5965–5971 (DOI: 10.1039/C2NR31648J)
- Unexpected formation of a cyclic vinylene sulfate in the synthesis of ethynylsubstituted acenes, B. Djukic, D.F. Perepichka*, Chem. Commun., 2012, 48, 6651–6653. (DOI: 10.1039/C2CC32805D)
- Maximizing field-effect mobility and solid-state luminescence in organic semiconductors, A. Dadvand, A.G. Moiseev, K. Sawabe, W.-H. Sun, B. Djukic, I. Chung, T. Takenobu, F. Rosei, D.F. Perepichka*, Angew. Chem.Int. Ed., 2012, 51, 3837-3841. (DOI: 10.1002/anie.201108184)
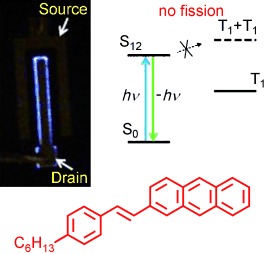
- Non-classical heteroacenes: synthesis and properties of anthra[2,3-c:6,7-c']dithiophene derivatives, B. Djukic, D.F. Perepichka*, Chem. Commun., 2011, 47, 12619-12621. (DOI: 10.1039/c1cc15623c)
- Halogen bonds as stabilizing interactions in a chiral self-assembled molecular monolayer, R. Gutzler, O. Ivasenko, C. Fu, J. L. Brusso, F. Rosei*, D. F. Perepichka*, Chem. Commun., 2011, 47, 9453–9455. (DOI: 10.1039/C1CC13114A)
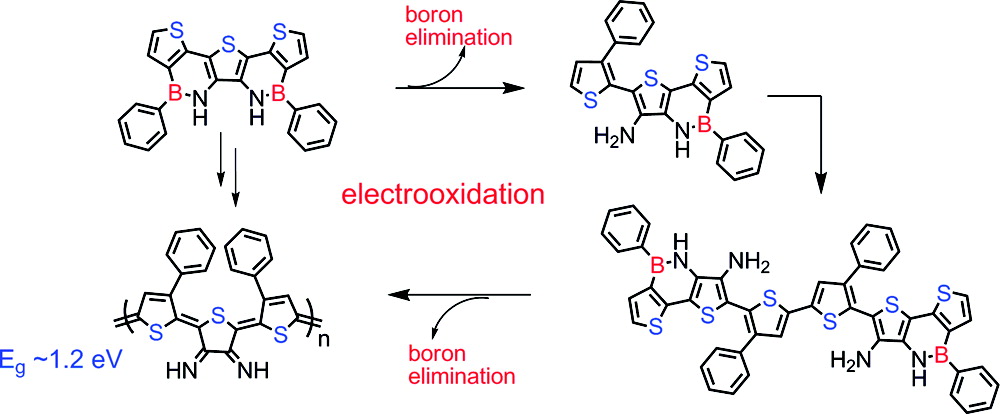
- Donor-Acceptor Intermediates and LowBandgap Polymers by Electropolymerization of Thienoazaborines, O. Lukoyanova, M. Lepeltier, M. Laferrière, D.F. Perepichka*, Macromolecules, 2011, 44, 4729–4734 (DOI: 10.1021/ma200503f)
- Towards “green” electronic materials. alpha-Oligofurans as Semiconductors, O. Gidron, A. Dadvand, Y.Sheynin, M. Bendikov*, D.F. Perepichka*, Chem. Commun., 2011, 47, 1976–1979 (DOI: 10.1039/C0CC04699J)
- New stable donor-acceptor dyads for molecular electronics, M. Kondratenko, A. Moiseev, D.F. Perepichka,* J. Mater. Chem., 2011, 21, 1470–1478 (DOI: 10.1039/C0JM02545C)
- Mastering fundamentals of supramolecular design with carboxylic acids. Common lessons from X-ray crystallography and scanning tunneling microscopy, O. Ivasenko, D.F. Perepichka* Chem. Soc. Rev., 2011, 40, 191–206. (DOI: 10.1039/C0CS00022A)
- New azaborine-thiophene heteroacenes, M. Lepeltier, O. Lukoyanova, A. Jacobson, D.F. Perepichka,* Chem. Commun., 2011, 46, 2007–2009. (DOI: 10.1039/C0CC01963A)
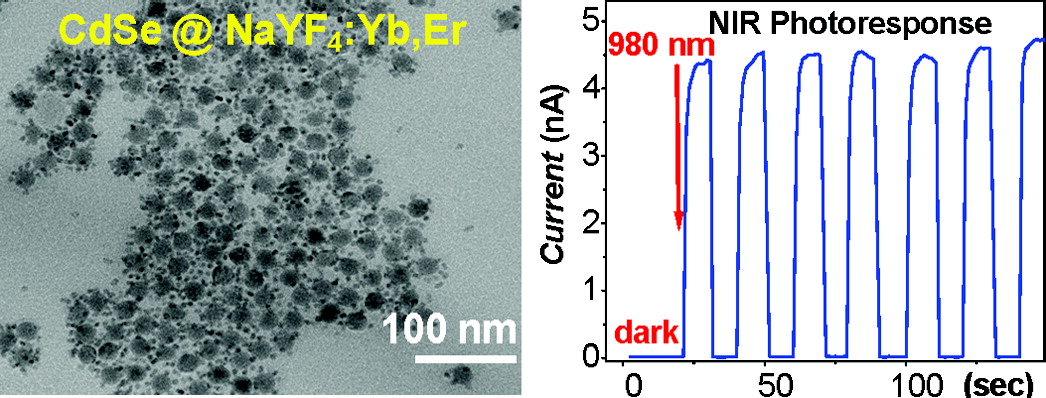
- NIR Photoresponse in New Up-converting CdSe/NaYF4:Yb,Er nano-heterostructures, C. Yan, A. Dadvand, F. Rosei, D. F. Perepichka,* J. Am. Chem. Soc., 2010, 132, 8868–8869. (DOI: 10.1021/ja103743t)
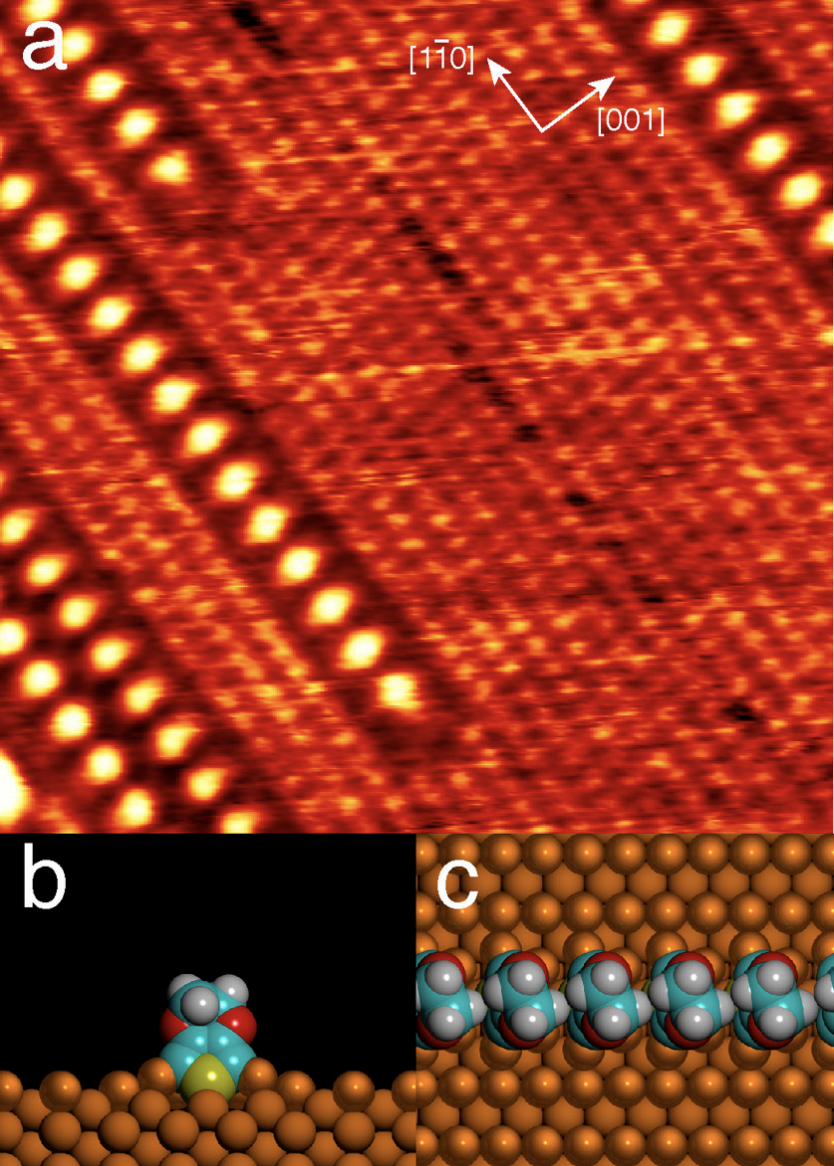
- Step-by-step growth of aligned polythiophene wires by surface-confined oligomerization, J.A. Lipton-Duffin, J.A. Miwa, M. Kondratenko, F.Cicoira, B. G. Sumpter, V. Meunier*, D.F. Perepichka*, F. Rosei*, Proc. Nat. Acad. Sci. USA, 2010, 107, 11200–11204. (DOI: 10.1073/pnas.1000726107)
- Highly emissive and electrochemically stable thienylene vinylene oligomers and copolymers: an unusual effect of alkylsulfanyl substituents, S. Jeeva, O. Lukoyanova, A. Karas, A. Dadvand, F. Rosei, D.F. Perepichka*, Adv. Funct. Mater., 2010, 20, 1661–1669. (DOI: 10.1002/adfm.200902300)
- Multiple NaNbO3/Nb2O5 nanotubes: a new class of semiconductor heterostructures, C. Yan, L. Nikolova, A. Dadvand, C. Harnagea, A. Sarkissian, D. F. Perepichka,* D. Xue,* F. Rosei*, Adv. Mater., 2010, 22, 1741-1744. (DOI: 10.1002/adma.200903589)

- Quasi temperature independent charge carrier mobility in hexagonal columnar mesophases of Hbonded benzotristhiophene derivative, A. Demenev, S. H. Eichhorn*, T. Taerum, D. F. Perepichka*, S. Patwardhan, F. C. Grozema*, L.D.A. Siebbeles, Chem. Mater., 2010, 22, 1420-1428. (DOI: 10.1021/cm902453z)

Before 2010
56) J. M. MacLeod, O. Ivasenko, C. Fu, T. Taerum, F. Rosei*, D.F.Perepichka*, Supramolecular ordering in
oligothiophene-fullerene monolayers studied by STM, J. Am. Chem. Soc. 2009, 131, 16844–16850.
55) T. Taerum, O. Lukoyanova, R. Wylie, D. F. Perepichka*, Synthesis, polymerization and unusual properties
of new star-shaped thiophene oligomers, Org. Lett. 2009, 11, 3230–3233.
54) M. Lepeltier, J. Hiltz, T. Lockwood, F. Bélanger-Gariépy, D.F. Perepichka*, Towards crystal engineering of
solid state polymerization in dibromothiophenes, J. Mater. Chem. 2009, 19, 5167–5174.
53) F. Vetrone, F. Variola, P. Tambasco de Oliveira, S. F. Zalzal, J.-H. Yi, J. Sam, A. Sarkissian, D.F.
Perepichka, J. D. Wuest, F. Rosei, A. Nanci*, Nanoscale oxidative patterning of metallic surfaces to
modulate cell activity and fate, Nano Lett. 2009, 9, 659–665.
52) J. A. Lipton-Duffin, O. Ivasenko, D. F. Perepichka*, F. Rosei*, Synthesis of polyphenylene molecular wires
by surface confined polymerization, Small 2009, 5, 592–597
51) F. Variola, F. Vetrone, L. Richert, P. Jedrzejowski, J.H. Yi, S. Zalzal, S. Clair, A. Sarkissian, D.F. Perepichka,
J.D. Wuest, F. Rosei, A. Nanci*, Improving biocompatibility of materials by nanoscale modification of
surfaces: an overview of strategies, fabrication methods and challenges, Small, 2009, 5, 996–1006 (refereed
Review)
50) D. F. Perepichka*, F. Rosei*, Extending polymer conjugation in the second dimension, Science 2009, 323,
216–217. (refereed Highlight)
49) O. Ivasenko, J. M. MacLeod, К. Chernichenko, E. Balenkova, R. V. Shpachenko, V. G. Nenajdenko, F. Rosei*,
D. F. Perepichka*, Supramolecular assembly of heterocirculenes in 2D and 3D, Chem. Commun., 2009,
1192–1194.
48) A. Dadvand, F. Cicoira, K. Yu. Chernichenko, E. S. Balenkova, R. M. Osuna, F. Rosei, V. G. Nenajdenko, D.
F. Perepichka*, Heterocirculenes as a new class of organic semiconductors, Chem. Commun. 2008, 5354–
5356.
47) J. A. Miwa, F. Cicora, J. Lipton-Duffin, D. F. Perepichka, C. Santato, F. Rosei*, Self-assembly of rubrene on
Cu(111), Nanotechnology 2008, 19, 424021.
46) J. A. Miwa, F. Cicoira, S. Bedwani, J. Lipton-Duffin, D. F. Perepichka, A. Rochefort, F. Rosei*, Self-assembly
of rubrene on copper surfaces, J. Phys. Chem. C. 2008, 112, 10214–10221.
45) S. Clair, F. Variola, M. Kondratenko, P. Jedrzejowski, A. Nanci, F. Rosei*, D.F. Perepichka*, Self-assembled
monolayer of alkanephosphoric acid on nanotextured Ti, J. Chem. Phys. 2008, 128, 144705.
44) J. L. Brusso, O. Hirst, A. Dadvand, S. Ganesan, F. Cicoira, C. M. Robertson, R. T. Oakley, F. Rosei, D. F.
Perepichka*, A New Structural Motif in Thienoacene Semiconductors: Synthesis, Structure and Properties of
Tetrathienoanthracene Isomers, Chem. Mater. 2008, 20, 2484–2494.
43) D. F. Perepichka*, I. F. Perepichka*, O. Ivasenko, A J. Moore,M. R. Bryce*, L. G. Kuz’mina, A.S. Batsanov,
N.I. Sokolov, Combining High Electron Affinity and Intramolecular Charge Transfer in Nitrofluorene – 1,3-
Dithiole Push-Pull Diads, Chem. Eur. J. 2008, 14, 2757–2770.
8 Perepichka
42) F. Cicoira, C. Santato, A. Dadvand, C. Harnagea, A. Pignolet, P. Bellutti, Z. Xiang, F. Rosei*, H. Meng*, D.F.
Perepichka*, Environmentally stable organic light emitting field effect transistors based on 2-(4-
pentylstyryl)tetracene, J. Mater. Chem. 2008, 18, 158–161.
41) F. Cicoira, J. A. Miwa, D. F. Perepichka, F. Rosei*, Molecular assembly of rubrene on a metal/metal oxide
nanotemplate, J. Phys. Chem. A 2007, 111, 12647–12678.
40) K.G. Nath, O. Ivasenko, J.L. Macleod, J.A. Miwa, J.D. Wuest, A. Nanci, D.F. Perepichka*, F. Rosei*, Crystal
engineering in two dimensions: an approach to molecular nanopatterning, J. Phys. Chem. C 2007, 111, 16996–
1700.
39) J. M. Macleod, O. Ivasenko, D. F. Perepichka*, F. Rosei*, Stabilization of exotic minority phases in a
multicomponent self-assembled molecular network, Nanotechnology 2007, 18, 424031.
38) D.F. Perepichka*, F. Rosei*, Metal nanoparticles: from “artificial atoms” to “artificial molecules”, Angew.
Chem. Int. Ed. 2007, 46, 6006–6008. (refereed Highlight)
37) K.G. Nath, O. Ivasenko, J. A. Miwa, H. Dang, J. D. Wuest, A. Nanci, D. F. Perepichka*, F. Rosei*, Rational
modulation of the periodicity in linear hydrogen-bonded assemblies of trimesic acid on surfaces, J. Am. Chem.
Soc. 2006, 128, 4212–4213. (top 1% in citation in chemistry)
36) S. Amriou, C. Wang, A. S. Batsanov, M. R. Bryce*, D. F. Perepichka, E. Ortí, R. Viruela, J. Vidal-Gancedo,
C. Rovira, The interplay of inverted redox potentials and aromaticity in the oxidized states of new electron
donors, Chem. Eur. J. 2006, 12, 3389–3400.
35) Z. Wei, M. Kondratenko, L.H. Dao, D.F. Perepichka*, Rectifying diodes from asymmetrically functionalized
single wall carbon nanotubes, J. Am. Chem. Soc. 2006, 128, 3134–3135.
34) D.F. Perepichka*, F. Rosei*, Silicon nanotubes, Small 2006, 2, 22–25. (refereed Highlight)
33) D.F. Perepichka*, M. Kondratenko, M.R. Bryce, Self-assembled monolayers of strong electron acceptors:
polynitrofluorenes on gold and platinum, Langmuir 2005, 21, 8824–8831.
32) Md.B.Zaman, D.F.Perepichka*, A new simple synthesis of poly(thiophene-methine)s, Chem. Commun. 2005,
4187–4189.
31) I.F. Perepichka*, D.F. Perepichka*, H. Meng*, F. Wudl*, Light-emitting polythiophenes, Adv. Mater. 2005,
19, 2281–2305. (refereed Review)
30) D.F.Perepichka*, M.R.Bryce*, Molecules with exceptionally low HOMO–LUMO Gap, Angew. Chem. Int.
Ed. 2005, 44, 5370–5373. (refereed Highlight)
29) G.Ho, J.R.Heath*, M.Kondratenko, D.F.Perepichka*, K.Arseneault, M.Pezolet, M.R.Bryce, The first studies
of a tetrathiafulvalene-sigma-acceptor molecular rectifier, Chem. Eur. J. 2005, 11, 2914–2922. (cover page)
28) M.Bendikov*, F.Wudl*, D.F.Perepichka*, Molecular materials across fields: TTFs, fullerenes and acenes,
Chem. Rev. 2004, 104, 4891–4945. (refereed Review)
27) D.F.Perepichka*, F.Wudl*, S.R.Wilson*, Y.Sun, D.I.Schuster*, The dissolution of carbon nanotubes in
Aniline, Revisited, J. Mater. Chem. 2004, 14, 2479–2482.
26) H.Meng, D.F.Perepichka, M.Bendikov, F.Wudl*, G.Z.Pan, Y.Wenjiang, W.Dong, S.Brown, Solid-state
synthesis of a conducting polythiophene via an unprecedented heterocyclic coupling reaction, J. Am. Chem.
Soc. 2003, 125, 15151–15162.
25) D.F.Perepichka, M.R.Bryce*, C.Pearson, M.C.Petty, E.J.L.McInnes, J.P.Zhao, A covalent tetrathiafulvalene–
tetracyanoquinodimethane diad: extremely low HOMO–LUMO gap, thermoexcited electron transfer and high
quality Langmuir Blodgett films, Angew. Chem. Int. Ed. 2003, 42, 4635–4639. (inside cover)
24) A.S.Batsanov*, D.F.Perepichka, A 1:1 cocrystal of 2,7-dicyanomethylene-4,5-dinitrofluorene and
benzonitrile, Acta Cryst. 2003, E59, o1318–1320.
23) D.F. Perepichka, M. Bendikov, H. Meng, F. Wudl*, A one-step synthesis of a poly(iptycene) through an
unusual Diels-Alder cyclization/dechlorination of tetrachloropentacene, J. Am. Chem. Soc. 2003, 125, 10190–
10191.
22) M.R. Bryce, G. Cooke*, F.M.A. Duclairoir, P. John, D.F. Perepichka, N. Polwart, V.M. Rotello, J.F. Stoddart,
H.-R. Tseng, Electrochemically controllable surfaces with three-pole binding properties, J. Mater. Chem. 2003,
13, 2111–2117.
21) B. de Boer, H. Meng, D.F. Perepichka, J. Zheng, M.M. Frank, Y.J. Chabal, Z. Bao*, Synthesis and
characterization of conjugated mono- and dithiol oligomers and characterization of their self-assembled
monolayers, Langmuir 2003, 19, 4272–4284. (10 most requested in 2004 by CAS)
20) H. Meng, D. F. Perepichka, F.Wudl*, Facile solid state synthesis of a highly conducting
poly(ethylenedioxythiophene), Angew. Chem. Int. Ed. 2003, 42, 658–661. (VIP and cover page)
19) D.F.Perepichka, M.R.Bryce*, I.F.Perepichka, S.B.Lyubchik, N.Godbert, C.A.Christensen, A.S.Batsanov,
E.Levillain, E.J.L.McInnes, J.P.Zhao, The first observation of A D2+−sigma−A•- species: an unusual electrochemistry of
(pi-extended tetrathiafulvalene)–sigma–fluorene conjugate, J. Am. Chem. Soc. 2002, 124, 14227–14238.
18) M.Brettreich, M.Bendikov, S.Chaffins, D.F.Perepichka, O.Dautel, H.Duong, R.Helgeson, F.Wudl*, Synthesis,
X-ray structure, and properties of a tetra-benzannelated 1,2,4,5-cyclophane, Angew. Chem. Int. Ed. 2002, 41,
3688–3691.
17) D.F.Perepichka, M.R.Bryce*, A.S.Batsanov, E.J.L.McInnes, J.P.Zhao, R.D.Farley, Engineering a remarkably
low HOMO–LUMO gap by covalent linkage of a strong pi-donor and pi-acceptor, Chem. Eur. J. 2002, 8, 4656–
4669.
16) A.S.Batsanov*, D.F.Perepichka, Methoxycarbonylmethyl 2-methoxycarbonyl-3-hydroxybenzo[b]furan-6-
carboxylate, Acta Cryst. 2002, E58, o1227–1228.
15) L.G.Kuz’mina, I.F.Perepichka*, D.F.Perepichka, J.A.K.Howard, M.R.Bryce*, Supramolecular architecture
for two charge-transfer complexes based on 2,7-(X,X)-4,5-dinitro-9-dicyanomethylenefluorenes (X = NO2 or
CN) with tetrathiafulvalene, Cryst. Reports 2002, 251–261.
14) D.F.Perepichka, M.R.Bryce*, A.S.Batsanov, J.A.K.Howard, A.O.Cuello, M.Gray, V.M.Rotello,
Trialkyltetrathiafulvalene-sigma-tetracyanoanthraquinodimethane (R3TTF-sigma-TCNAQ) diads: synthesis,
intramolecular charge-transfer properties and X-ray crystal structure, J. Org. Chem. 2001, 66, 4517–4524.
13) A.S.Batsanov, J.C.Collings, J.A.K.Howard, T.B.Marder*, D.F.Perepichka, Arene–perfluoronaphthalene
interactions in crystal engineering. 5. Octafluoronaphthalene–tetrathiafuvalene (1/1), Acta Cryst. 2001, C57,
1306–1307.
12) D.F.Perepichka, I.F.Perepichka*, M.R.Bryce*, N.I.Sokolov, A.J.Moore, pi–Extended nitrofluorene-1,3-
dithiole chromophore: enhancing the photoresponse of holographic materials through the balance of
intramolecular charge transfer and electron affinity, J. Mater. Chem. 2001, 11, 1772–1774.
11) D.F.Perepichka, M.R.Bryce*, E.J.L.McInnes, J.P.Zhao, The first tetrathiafulvalene–σ–polynitrofluorene
diads: low HOMO–LUMO gap, amphoteric redox behavior & CT properties, Org. Lett. 2001, 3, 1431–1434.
10) D.F. Perepichka, I.F. Perepichka*, M.R. Bryce*, A.J. Moore, N.I. Sokolov, Push-pull dithiole – fluorene
acceptors as electron transport materials for holography, Synth. Met. 2001, 121, 1487–1488.
9) A.J.Moore, A.Chesney, M.R.Bryce*, A.S.Batsanov, J.F.Kelly, J.A.K.Howard, I.F.Perepichka, D.F.Perepichka,
G.Meshulam, G.Berkovich, Z.Kotler, R.Mazor, V.Khodorkovsky, Synthesis, structures and non-linear optical
properties of novel D–pi–A chromophores: intramolecular charge transfer from 1,3-dithiole or ferrocene
moieties to polynitrofluorene or dicyanomethylene moieties through conjugated spacers, Eur. J. Org. Chem.
2001, 2671–2687.
8) M.R.Bryce*, A.Green, A.J.Moore, D.F.Perepichka, A.S.Batsanov, J.A.K.Howard, I.Ledoux-Rak, M.González,
N.Martín, J.L.Segura, J.Garín, J.Orduna, R.Alcalá, B.Villacampa, Synthesis of conjugated tetrathiafulvalene
(TTF)–pi–acceptor molecules: intramolecular charge transfer and nonlinear optical properties, Eur. J. Org.
Chem., 2001, 1927–1935.
7) I.F.Perepichka*, D.F.Perepichka, S.B.Lyubchik, M.R.Bryce*, A.S.Batsanov, J.A.K.Howard, Electron
acceptors of the fluorene series. 13. 9-(5-Nitrofuran-2-ylidene)- and 9-(5-nitrothien-2-ylidene)-2,4,5,7-
tetranitrofluorenes: novel pi-extended electron acceptors. Synthesis, cyclic voltammetry, X-ray crystal
structures for the acceptor and its 4,5-dimethyltetrathiafulvalene complex, and the theoretical study, J. Chem.
Soc., Perkin Trans. 2, 2001, 1546–1551.
6) A.E.Jones, C.A.Christensen, D.F.Perepichka, A.S.Batsanov, A.Beeby, P.J.Low, M.R.Bryce*, A.W.Parker,
Photochemistry of the pi-extended 9,10-bis(1,3-dithiol-2-ylidene)-9,10-dihydroanthracene system: generation
and characterisation of the radical cation, dication and derived products, Chem. Eur. J., 2001, 7, 973–978.
10 Perepichka
5) D.F.Perepichka, I.F.Perepichka*, A.F.Popov, M.R.Bryce*, A.S.Batsanov, A.Chesney, J.A.K.Howard,
N.I.Sokolov, Electron Acceptors of the Fluorene Series. Part 12. 9-(Metalloceneylidene)nitrofluorene
derivatives of Fc–NF, NF–Fc–NF, and NF–Rc–NF types, and the vinylogues Fc–pi–NF: synthesis,
characterisation, intramolecular charge transfer, redox properties and X-ray structures for three
fluoreneferrocene derivatives, J. Organometal. Chem. 2001, 637–639, 445–462.
4) I. F. Perepichka*, D. F. Perepichka, M. R. Bryce*, A. Chesney, A. F. Popov, V. Khodorovsky, L. Shapiro, Z.
Kotler, Push-pull fluorene acceptors with ferrocene donor moiety, – Synth. Met. 1999, 102, 1558–1559.
3) D. D. Mysyk, I. F. Perepichka*, D. F. Perepichka, M. R. Bryce*, A. F. Popov, L. M. Goldenberg, A. J. Moore,
Electron acceptors of the fluorene series. 9. Derivatives of 9-(1,2-dithiol-3-ylidene), 9-(1,3-dithiol-2-ylidene)-
, and 9-(1,3-selenathiol-2-ylidene)fluorenes: synthesis, intramolecular charge transfer and redox properties, J.
Org. Chem. 1999, 64, 6937–6950.
2) I. F. Perepichka*, L. G. Kuz’mina, D. F. Perepichka, M. R. Bryce*, L. M. Goldenberg, A. F. Popov, J. A. K.
Howard, Electron acceptors of the fluorene series. 7. 2,7-Dicyano-4,5-dinitro-9-X-fluorenes: synthesis, cyclic
voltammetry, charge transfer complexation with N-propylcarbazole in solution and X-Ray crystal structures of
two tetrathiafulvalene complexes, J. Org. Chem. 1998, 63, 6484–6493.
1) I.F. Perepichka*, D.F. Perepichka, M.R. Bryce*, L.M. Goldenberg, L.G. Kuz’mina, A.F. Popov, A. Chesney,
A. J. Moore, J. A. K. Howard, N. I. Sokolov, Fluorene acceptors with intramolecular charge-transfer from 1,3-
dithiole donor moieties: novel electron transport materials, Chem. Commun. 1998, 819–820.